Coordination Number
In coordination chemistry, the coordination number is the number of ligands attached to the central ion (more specifically, the number of donor atoms). Coordination numbers are normally between two and nine. The number of bonds depends on the size, charge, and electron configuration of the metal ion and the ligands.
Typically the chemistry of complexes is dominated by interactions between s and p molecular orbitals of the ligands and the d orbitals of the metal ions. The s, p, and d orbitals of the metal can accommodate 18 electrons. The maximum coordination number for a certain metal is thus related to the electronic configuration of the metal ion (specifically, the number of empty orbitals) and to the ratio of the size of the ligands and the metal ion. Large metals and small ligands lead to high coordination numbers (e.g., [Mo(CN)8]4−). Small metals with large ligands lead to low coordination numbers (e.g., Pt[P(CMe3)]2). Due to their large size, lanthanides, actinides, and early transition metals tend to have high coordination numbers.
Ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. Virtually every molecule and every ion can serve as a ligand for (or coordinate to) metals. Denticity refers to the number of times a ligand bonds to a metal through donor atoms. Many ligands are capable of binding metal ions through multiple sites, usually because the ligands have lone pairs on more than one atom.
Monodentate ligands include virtually all anions and all simple Lewis bases. Thus, the halides and pseudohalides are important anionic ligands. Ammonia, carbon monoxide, and water are particularly common charge-neutral ligands. Simple organic species are also very common. All unsaturated molecules are also ligands, utilizing their π-electrons in forming the coordinate bond. Also, metals can bind to the σ bonds in, for example, silanes, hydrocarbons, and dihydrogen.
Ligands that bind via more than one atom are often termed polydentate or chelating. A ligand that binds through two sites is classified as bidentate, and three sites as tridentate. Chelating ligands are commonly formed by linking donor groups via organic linkers. A classic bidentate ligand is ethylenediamine, which is derived by the linking of two ammonia groups with an ethylene (-CH2CH2-) linker. A classic example of a polydentate ligand is the hexadentate chelating agent EDTA, which is able to bond through six sites, completely surrounding some metals.
There are several types of polydentate ligands which can be characterized based on how they interact with the central ion. For example, trans-spanning ligands are bidentate ligands that can span coordination positions on opposite sides of a coordination complex. Ambidentate ligands can attach to the central atom in two places but not both. A bridging ligand links two or more metal centers. Changing the size and electronic properties of ligands can be used to control catalysis of the central ion and stabilize unusual coordination sites.
Geometries
Different ligand structural arrangements result from the coordination number. Most structures follow the pattern as if the central atom were in the middle and the corners of that shape are the locations of the ligands. These shapes are defined by orbital overlap between ligand and metal orbitals and ligand-ligand repulsions, which tend to lead to certain regular geometries. However, there are many cases that deviate from regular geometry. For example, ligands of different sizes and with different electronic effects often result in irregular bond lengths.
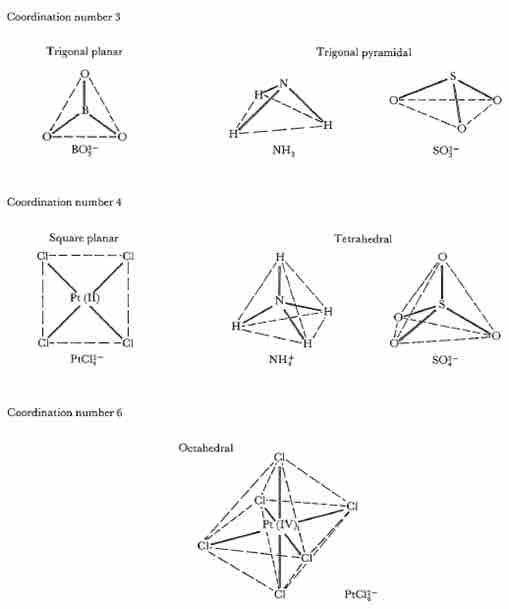
Geometry of atoms around central atoms with coordination numbers
Geometry of atoms around central atoms with coordination numbers 3, 4, and 6. If L is any peripheral atom and M is the central atom, then the bond angle L - M - L is 120° for trigonal planar, 109.5° for tetrahedral, and typically around 109.5° for trigonal pyramidal geometries. Square planar and octahedral geometries have two L - M - L angles, 90° and 180°.