Hydrogenation Reactions
Hydrogenation refers to the treatment of substances with molecular hydrogen (H2), adding pairs of hydrogen atoms to compounds (generally unsaturated compounds). These usually require a catalyst for the reaction to occur under normal conditions of temperature and pressure. Most hydrogenation reactions use gaseous hydrogen as the hydrogen source, but alternative sources have been developed. The reverse of hydrogenation, where hydrogen is removed from the compounds, is known as dehydrogenation. Hydrogenation differs from protonation or hydride addition because in hydrogenation the products have the same charge as the reactants.
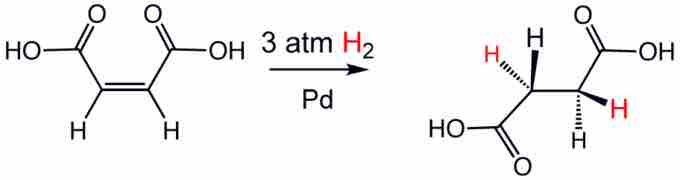
Hydrogenation
Hydrogen can be added across a double bond—such as the olefin in maleic acid shown—by utilizing a catalyst, such as palladium.
Hydrogenation reactions generally require three components: the substrate, the hydrogen source, and a catalyst. The reaction is carried out at varying temperatures and pressures depending on the catalyst and substrate used. The hydrogenation of an alkene produces an alkane. The addition of hydrogen to compounds happens in a syn addition fashion, adding to the same face of the compound and entering from the least hindered side. Generally, alkenes will convert to alkanes, alkynes to alkenes, aldehydes and ketones to alcohols, esters to secondary alcohols, and amides to amines via hydrogenation reactions.
Catalysts of Hydrogenation
Generally, hydrogenation reactions will not occur between hydrogen and organic compounds below 480 degrees Celsius without metal catalysts. Catalysts are responsible for binding the H2 molecule and facilitating the reaction between the hydrogen and the substrate. Platinum, palladium, rhodium, and ruthenium are known to be active catalysts which can operate at lower temperatures and pressures. Research is ongoing to procure non-precious metal catalysts which can produce similar activity at lower temperatures and pressures. Nickel-based catalysts, such as Raney nickel, have been developed, but still require high temperatures and pressures.
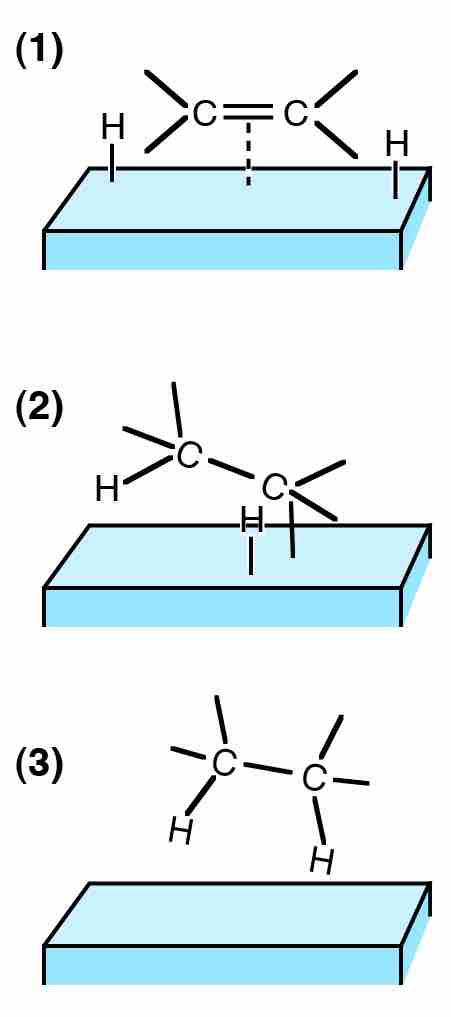
Heterogeneous Catalysis
The hydrogenation of ethylene (C2H4) on a solid support is an example of heterogeneous catalysis.
Catalysts can be divided into two categories: homogeneous or heterogeneous catalysts. Homogeneous catalysts are soluble in the solvent that contains the unsaturated substrate. Heterogeneous catalysts are found more commonly in industry, and are not soluble in the solvent containing the substrate. Often, heterogeneous catalysts are metal-based and are attached to supports based on carbon or oxide. The choice of support for these materials is important, as the supports can affect the activity of the catalysts. Hydrogen gas is the most common source of hydrogen used and is commercially available.
Hydrogenation is an exothermic reaction, releasing about 25 kcal/mol in the hydrogenation of vegetable oils and fatty acids. For heterogenous catalysts, the Horiuti-Polanyi mechanism explains how hydrogenation occurs. First, the unsaturated bond binds to the catalyst, followed by H2 dissociation into atomic hydrogen onto the catalyst. Then one atom of hydrogen attaches to the substrate in a reversible step, followed by the addition of a second atom, rendering the hydrogenation process irreversible. For homogeneous catalysis, the metal binds to hydrogen to give a dihydride complex via oxidative addition. The metal binds the substrate and then transfers one of the hydrogen atoms from the metal to the substrate via migratory insertion. The second hydrogen atom from the metal is transferred to the substrate with simultaneous dissociation of the newly formed alkane via reductive elimination.
Industrial Uses of Hydrogenation Reactions
Heterogeneous catalytic hydrogenation is very important in industrial processes. In petrochemical processes, hydrogenation is used to saturate alkenes and aromatics, making them less toxic and reactive. Hydrogenation is also important in processing vegetable oils because most vegetable oils are derived from polyunsaturated fatty acids. Partial hydrogenation reduces most, but not all, of the carbon-carbon double bonds, making them better for sale and consumption. The degree of saturation of fats changes important physical properties such as the melting range of the oils; an example of this is how liquid vegetable oils become semi-solid at various temperatures.
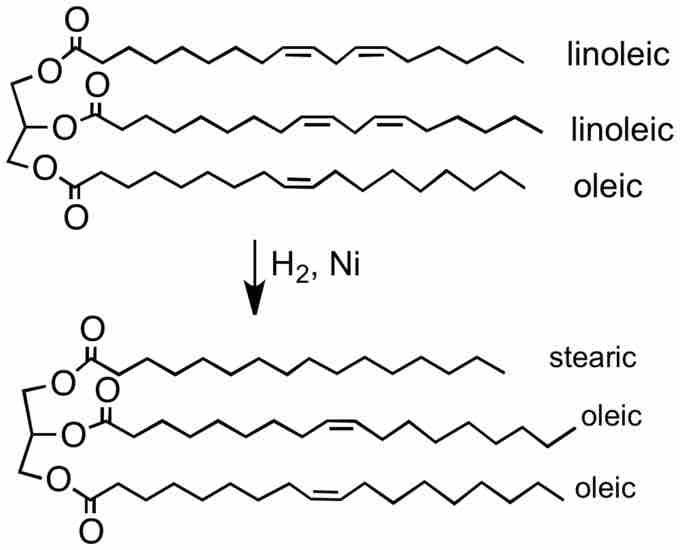
Partial hydrogenation in margarine
Margarine is a semi-solid butter substitute created from vegetable oil, which is typically unsaturated and therefore liquid at room temperature. The process of partial hydrogenation adds hydrogen atoms and reduces the double bonds in the fatty acids, creating a semi-solid vegetable oil at room temperature.
Incomplete hydrogenation of the double bonds has health implications; some double bonds can isomerize from the cis to the trans state. This isomerization occurs because the trans configuration has lower energy than the cis configuration. The trans isomers have been implicated in contributing to pathological blood circulatory system conditions (i.e.,atherosclerosis and heart disease).