Bond vs Molecular Polarity
Polarity refers to the separation of charge that creates permanent positive and negative 'electric poles.' This concept can be applied in two contexts:
- Bond polarity: when atoms from different elements are covalently bonded, the shared pair of electrons will be attracted more strongly to the atom with the higher electronegativity. As a result, the electrons will not be shared equally. Such bonds are said to be 'polar' and possess partial ionic character.
- Molecular polarity: when an entire molecule, which can be made out of several covalent bonds, has a net polarity, with one end having a higher concentration of negative charge and another end having a surplus of positive charge. A polar molecule acts as an electric dipole which can interact with electric fields that are created artificially, or that arise from interactions with nearby ions or other polar molecules.
Dipole Moment
Dipoles are conventionally represented as arrows pointing in the direction of the negative end. The strength of a dipole's interaction with an electric field is given by the electric dipole moment of the bond or molecule. The dipole moment is calculated by evaluating the product of the magnitude of separated charge, q, and the bond length, r:
In SI units, q is expressed in coulombs and r in meters, so μ has the dimensions of
The Debye unit, D, is commonly used to express dipole moments.
Determining a Molecule's Dipole Moment
In molecules containing more than one polar bond, the molecular dipole moment is just the vector addition of the individual bond dipole moments. Being vectors, these can reinforce or cancel each other depending on the geometry of the molecule. Therefore, it is possible for molecules containing polar bonds to be nonpolar overall, as in the example of carbon dioxide.
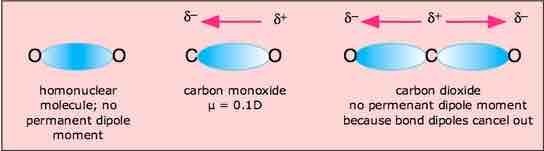
Molecular dipole moment of carbon dioxide
The linear shape of the CO2 molecule results in the canceling of the dipole moments of the two polar C=O bonds. The net, molecular dipole moment of CO2 is therefore zero, and the molecule is nonpolar.
H2O, by contrast, has a very large molecular dipole moment which results from the two polar H–O bonds forming an angle of 104.5° between them. The water molecule, therefore, is polar.
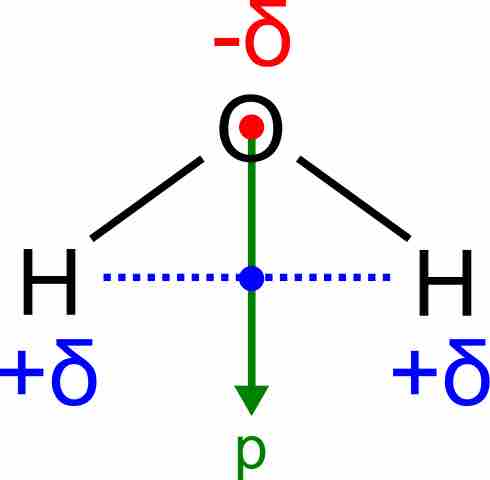
Dipole moment of a water molecule
Water has a very large dipole moment which results from the two polar H–O bonds oriented at an angle of 104.5° with respect to each other. The bond dipoles add up to create a molecular dipole (indicated by the green arrow).