Concept
Version 10
Created by Boundless
Bond Polarity
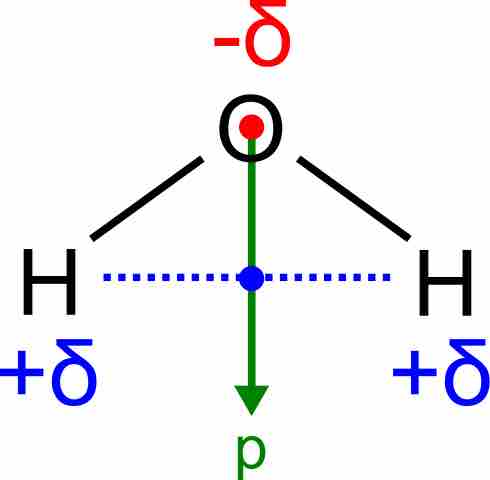
Dipole moment of a water molecule
Water has a very large dipole moment which results from the two polar H–O bonds oriented at an angle of 104.5° with respect to each other. The bond dipoles add up to create a molecular dipole (indicated by the green arrow).
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"490px-Dipole_Water.svg.png."
https://commons.wikimedia.org/wiki/File:Dipole_Water.svg
Wikimedia Commons
CC BY 4.0.