Concept
Version 10
Created by Boundless
Bond Polarity
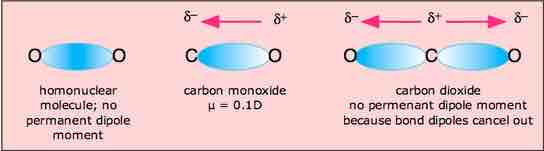
Molecular dipole moment of carbon dioxide
The linear shape of the CO2 molecule results in the canceling of the dipole moments of the two polar C=O bonds. The net, molecular dipole moment of CO2 is therefore zero, and the molecule is nonpolar.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: