A gas evolution reaction is a chemical process that produces a gas, such as oxygen or carbon dioxide. In the following examples, an acid reacts with a carbonate, producing salt, carbon dioxide, and water, respectively.
Nitric acid reacts with sodium carbonate to form sodium nitrate, carbon dioxide, and water:
Sulfuric acid reacts with calcium carbonate to form calcium sulfate, carbon dioxide, and water:
Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water:
The following setup demonstrates this type of reaction:
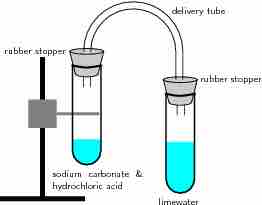
Reaction of acids with carbonates
In this reaction setup, lime water is poured into one of the test tubes and sealed with a stopper. A small amount of hydrochloric acid is carefully poured into the remaining test tube. A small amount of sodium carbonate is added to the acid, and the tube is sealed with a rubber stopper. The two tubes are connected. As a result of the acid-carbonate reaction, carbon dioxide is produced and the lime water turns milky.
The test tube on the right contains limewater (a solution of calcium hydroxide, Ca(OH)2). On the left, a solution of hydrochloric acid has been added to a solution of sodium carbonate to generate
When this experiment is repeated with nitric or sulfuric acid instead of HCl, it yields the same results: the clear limewater turns milky, indicating the production of carbon dioxide.
Oxidation of Metals in Acidic Solution
The oxidation of a metal in acidic solution will yield a metal salt and hydrogen gas:
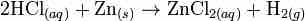
Acid and metal reaction
Hydrochloric acid oxidizes zinc to produce an aqueous metal salt and hydrogen gas bubbles.
Recall that oxidation refers to a loss of electrons, and reduction refers to the gain of electrons. In the above redox reaction, neutral zinc is oxidized to Zn2+, and the acid, H+, is reduced to H2(g). The oxidation of metals by strong acids is another common example of a gas evolution reaction.