Section 3
Acid-Base Reactions
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts
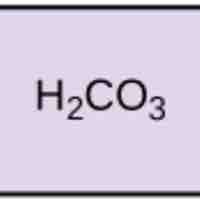
pH, Buffers, Acids, and Bases
Acids dissociate into H+ and lower pH, while bases dissociate into OH- and raise pH; buffers can absorb these excess ions to maintain pH.
Brønsted Acids and Bases
A Brønsted acid is any species capable of donating a proton; a Brønsted base is any capable of accepting a proton.
Acid-Base Titrations
Acid-base titration can determine the concentrations of unknown acid or base solutions.
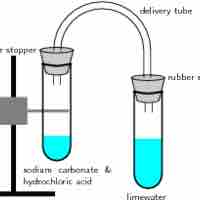
Gas Evolution Reactions
A gas evolution reaction is a chemical process that produces a gas, such as oxygen or carbon dioxide.