Concept
Version 11
Created by Boundless
Gas Evolution Reactions
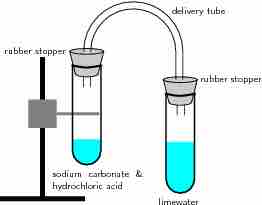
Reaction of acids with carbonates
In this reaction setup, lime water is poured into one of the test tubes and sealed with a stopper. A small amount of hydrochloric acid is carefully poured into the remaining test tube. A small amount of sodium carbonate is added to the acid, and the tube is sealed with a rubber stopper. The two tubes are connected. As a result of the acid-carbonate reaction, carbon dioxide is produced and the lime water turns milky.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Free High School Science Texts Project, Types of Reactions: Acid-Base Reactions. September 30, 2012."
http://cnx.org/content/m39088/latest/
OpenStax CNX
CC BY 3.0.