The induction of a signaling pathway depends on the modification of a cellular component by an enzyme. There are numerous enzymatic modifications that can occur which are recognized in turn by the next component downstream.
One of the most common chemical modifications that occurs in signaling pathways is the addition of a phosphate group (PO4–3) to a molecule such as a protein in a process called phosphorylation . The phosphate can be added to a nucleotide such as GMP to form GDP or GTP. Phosphates are also often added to serine, threonine, and tyrosine residues of proteins where they replace the hydroxyl group of the amino acid. The transfer of the phosphate is catalyzed by an enzyme called a kinase. Various kinases are named for the substrate they phosphorylate. Phosphorylation of serine and threonine residues often activates enzymes. Phosphorylation of tyrosine residues can either affect the activity of an enzyme or create a binding site that interacts with downstream components in the signaling cascade. Phosphorylation may activate or inactivate enzymes; the reversal of phosphorylation, dephosphorylation by a phosphatase, will reverse the effect.
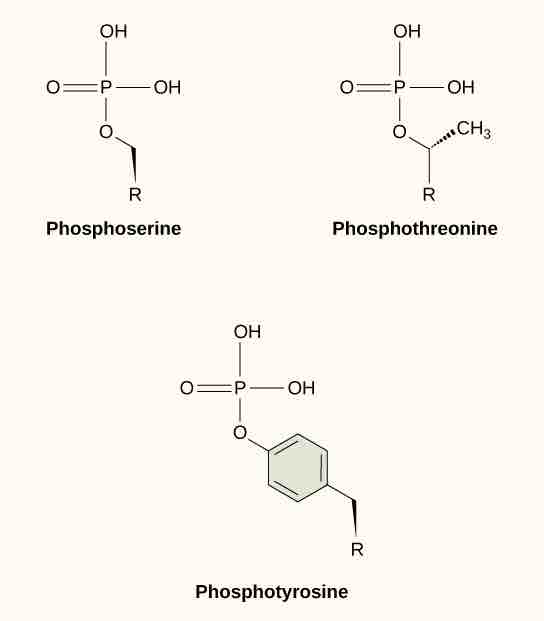
Example of phosphorylation
In protein phosphorylation, a phosphate group (PO4-3 ) is added to residues of the amino acids serine, threonine, and tyrosine.
The activation of second messengers is also a common event after the induction of a signaling pathway. They are small molecules that propagate a signal after it has been initiated by the binding of the signaling molecule to the receptor. These molecules help to spread a signal through the cytoplasm by altering the behavior of certain cellular proteins.
Calcium ion is a widely-used second messenger. The free concentration of calcium ions (Ca2+) within a cell is very low because ion pumps in the plasma membrane continuously use adenosine-5'-triphosphate (ATP) to remove it. For signaling purposes, Ca2+ is stored in cytoplasmic vesicles, such as the endoplasmic reticulum, or accessed from outside the cell. When signaling occurs, ligand-gated calcium ion channels allow the higher levels of Ca2+ that are present outside the cell (or in intracellular storage compartments) to flow into the cytoplasm, which raises the concentration of cytoplasmic Ca2+. The response to the increase in Ca2+ varies, depending on the cell type involved. For example, in the β-cells of the pancreas, Ca2+ signaling leads to the release of insulin, whereas in muscle cells, an increase in Ca2+ leads to muscle contractions.
Another second messenger utilized in many different cell types is cyclic AMP (cAMP). Cyclic AMP is synthesized by the enzyme adenylyl cyclase from ATP . The main role of cAMP in cells is to bind to and activate an enzyme called cAMP-dependent kinase (A-kinase). A-kinase regulates many vital metabolic pathways. It phosphorylates serine and threonine residues of its target proteins, activating them in the process. A-kinase is found in many different types of cells; the target proteins in each kind of cell are different. Differences give rise to the variation of the responses to cAMP in different cells.
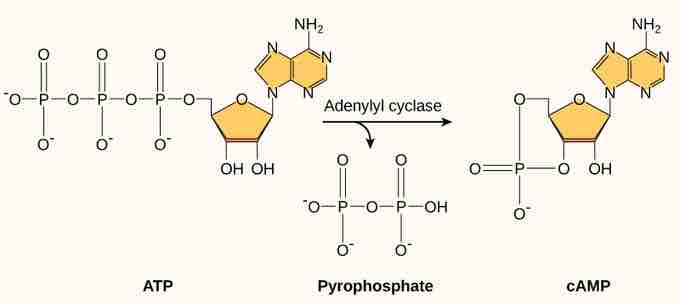
Example of cAMP as a second messenger
This diagram shows the mechanism for the formation of cyclic AMP (cAMP). cAMP serves as a second messenger to activate or inactivate proteins within the cell. Termination of the signal occurs when an enzyme called phosphodiesterase converts cAMP into AMP.
Present in small concentrations in the plasma membrane, inositol phospholipids are lipids that can also be converted into second messengers. Because these molecules are membrane components, they are located near membrane-bound receptors and can easily interact with them. Phosphatidylinositol (PI) is the main phospholipid that plays a role in cellular signaling. Enzymes known as kinases phosphorylate PI to form PI-phosphate (PIP) and PI-bisphosphate (PIP2).