Aluminium chloride
Background to the schools Wikipedia
This Schools selection was originally chosen by SOS Children for schools in the developing world without internet access. It is available as a intranet download. See http://www.soschildren.org/sponsor-a-child to find out about child sponsorship.
| Aluminium chloride | |
|---|---|
 |
|
 |
|
|
Aluminium(III) chloride |
|
|
Other names
Aluminium trichloride |
|
| Identifiers | |
| Properties | |
| Molecular formula | AlCl3 |
| Molar mass | 133.34 g mol−1 (anhydrous) 241.432 g mol−1 (hexahydrate) |
| Appearance | Pale yellow solid, hygroscopic. |
| Density | 2.48 g cm−3 |
| Melting point |
190 °C 463 K) |
| Boiling point |
178 °C (451 K) ( subl) |
| Solubility in water | 43.9 g/100 ml (0°C) 44.9 g/100 ml (10°C) 45.8 g/100 ml (20°C) 46.6 g/100 ml (30°C) 47.3 g/100 ml (40°C) 48.1 g/100 ml (60°C) 48.6 g/100 ml (80°C) 49 g/100 ml (100°C) |
| Structure | |
| Crystal structure | 6-coordinate layer lattice |
| Coordination geometry |
Octahedral (solid) Tetrahedral (liquid) |
| Molecular shape | Trigonal planar ( monomeric vapour) |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Corrosive (C) |
| R-phrases | R34 |
| S-phrases | (S1/2), S7/8, S28, S45 |
| Related compounds | |
| Other anions | Aluminium fluoride Aluminium bromide Aluminium iodide |
| Other cations | Boron trichloride Gallium(III) chloride Indium(III) chloride Thallium(III) chloride Magnesium chloride |
| Related Lewis acids | Iron(III) chloride Boron trifluoride |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
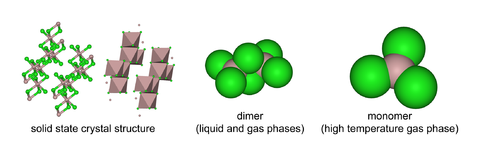
Aluminium chloride (AlCl3) is a compound of aluminium and chlorine. The solid has a low melting and boiling point, and is covalently bonded. It sublimes at 178 °C. Molten AlCl3 conducts electricity poorly, unlike more ionic halides such as sodium chloride. It exists in the solid state as a six-coordinate layer lattice.
AlCl3 adopts the "YCl3" structure, featuring Al3+ cubic close packed layered structure. In contrast, AlBr3 has a more molecular structure, with the Al3+ centers occupying adjacent tetrahedral holes of the close-packed framework of Br− ions. Upon melting AlCl3 gives the dimer Al2Cl6, which can vaporise. At higher temperatures this Al2Cl6 dimer dissociates into trigonal planar AlCl3, which is structurally analogous to BF3.
Aluminium chloride is highly deliquescent, and it can explode upon abrupt contact with water because of the high heat of hydration. Aqueous solutions of AlCl3 are ionic and thus conduct electricity well. Such solutions are found to be acidic, indicative of partial hydrolysis of the Al3+ ion. The reactions can be described (simplified) as:
- [Al(H2O)6]3+ + H2O ⇌ [Al(OH)(H2O)5]2+ + H3O+
AlCl3 is probably the most commonly used Lewis Acid and also one of the most powerful. It finds widespread application in the chemical industry as the classic catalyst for Friedel-Crafts reactions, both acylations and alkylations. It also finds use in polymerization and isomerization reactions of hydrocarbons. Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapour phase.
Chemical properties
Aluminium chloride is a powerful Lewis acid, capable of forming stable Lewis acid-base adducts with even weak Lewis bases such as benzophenone or mesitylene. Not surprisingly it forms AlCl4− in the presence of chloride ions.
In water, partial hydrolysis forms HCl gas or H3O+, as described in the overview above. Aqueous solutions behave similarly to other aluminium salts containing hydrated Al3+ ions, giving a gelatinous precipitate of aluminium hydroxide upon reaction with the correct quantity of aqueous sodium hydroxide:
AlCl3( aq) + 3 NaOH(aq) → Al(OH)3(s) + 3NaCl(aq)
Preparation
Aluminium chloride is manufactured on a large scale by the exothermic reaction of aluminium metal with chlorine or hydrogen chloride.
- 2 Al + 3 Cl2 → 2 AlCl3
- 2 Al + 6 HCl → 2 AlCl3 + 3 H2
Hydrated forms are prepared by dissolving aluminium oxides with hydrochloric acid.
Uses
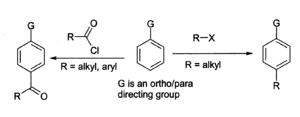
The Friedel-Crafts reaction is the major use for aluminium chloride, for example in the preparation of anthraquinone (for the dyestuffs industry) from benzene and phosgene. In the general Friedel-Crafts reaction, an acyl chloride or alkyl halide reacts with an aromatic system as shown:
With benzene derivatives, the major product is the para isomer. The alkylation reaction has many associated problems, such as in Friedel-Crafts, so it is less widely used than the acylation reaction. For both reactions, the aluminium chloride, as well as other materials and the equipment, must be moderately dry, although a trace of moisture is necessary for the reaction to proceed. A general problem with the Friedel-Crafts reaction is that the aluminium chloride "catalyst" needs to be present in full stoichiometric quantities in order for the reaction to go to completion, because it complexes strongly with the products (see chemical properties above). This makes it very difficult to recycle, so it must be destroyed after use, generating a large amount of corrosive waste. For this reason chemists are examining the use of more environmentally benign catalysts such as ytterbium(III) triflate or dysprosium(III) triflate, which can be recycled.
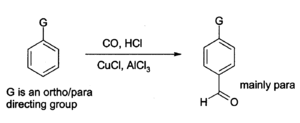
Aluminium chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gatterman-Koch reaction which uses carbon monoxide, hydrogen chloride and a copper(I) chloride co-catalyst):
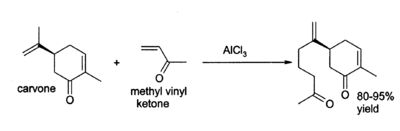
Aluminium chloride finds a wide variety of other applications in organic chemistry. For example, it can catalyse the " ene reaction", such as the addition of 3-buten-2-one (methyl vinyl ketone) to carvone:
AlCl3 is also widely used for polymerization and isomerization reactions of hydrocarbons. Important examples include the manufacture of ethylbenzene, which used to make styrene and thus polystyrene, and also production of dodecylbenzene, which is used for making detergents.
Aluminium chloride combined with aluminium in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g. bis(benzene)chromium, from certain metal halides via the so-called Fischer-Hafner synthesis.
Aluminium chloride, often in the form of derivatives such as aluminium chlorohydrate, is a common component in antiperspirants at low concentrations. Hyperhidrosis sufferers need a much higher concentration (15% or higher), sold under such brand names as Drysol®, Maxim®, Odaban®, CertainDri®, B+Drier® and Driclor®.
Precautions
Anhydrous AlCl3 reacts vigorously with water and bases, so suitable precautions are required. Hydrated salts are less problematic.