Metabolism in humans is the conversion of food into energy, which is then used by the body to perform activities. It is an example of the first law of thermodynamics in action. Considering the body as the system of interest, we can use the first law to examine heat transfer, doing work, and internal energy in activities ranging from sleep to heavy exercise. For example, one major factor in such activities is body temperature—normally kept constant by heat transfer to the surroundings, meaning that Q is negative (i.e., our body loses heat). Another factor is that the body usually does work on the outside world, meaning that W is positive. Thus, in such situations the body loses internal energy, since ΔU=Q−W is negative.
Eating
Now consider the effects of eating. The body metabolizes all the food we consume. Eating increases the internal energy of the body by adding chemical potential energy. In essence, metabolism uses an oxidation process in which the chemical potential energy of food is released. This implies that food input is in the form of work. Food energy is reported in a special unit, known as the Calorie. This energy is measured by burning food in a calorimeter, which is how the units are determined.
Catabolism and Anabolism
Catabolism is the pathway that breaks down molecules into smaller units and produces energy. Anabolism is the building up of molecules from smaller units. Anabolism uses up the energy produced by the catabolic break down of your food to create molecules more useful to your body.
Internal energy
Our body loses internal energy, and there are three places this internal energy can go—to heat transfer, to doing work, and to stored fat (a tiny fraction also goes to cell repair and growth). As shown in Fig 1 heat transfer and doing work take internal energy out of the body, and then food puts it back. If you eat just the right amount of food, then your average internal energy remains constant. Whatever you lose to heat transfer and doing work is replaced by food, so that, in the long run, ΔU=0. If you overeat repeatedly, then ΔU is always positive, and your body stores this extra internal energy as fat. The reverse is true if you eat too little. If ΔU is negative for a few days, then the body metabolizes its own fat to maintain body temperature and do work that takes energy from the body. This process is how dieting produces weight loss.
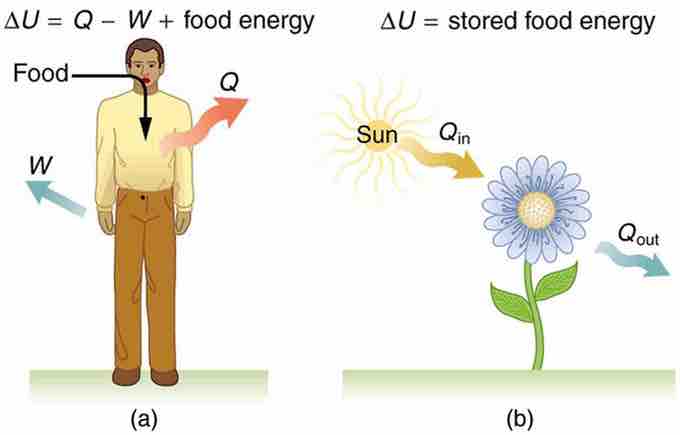
Metabolism
(a) The first law of thermodynamics applied to metabolism. Heat transferred out of the body (Q) and work done by the body (W) remove internal energy, while food intake replaces it. (Food intake may be considered as work done on the body. ) (b) Plants convert part of the radiant heat transfer in sunlight to stored chemical energy, a process called photosynthesis.
Metabolism
Life is not always this simple, as any dieter knows. The body stores fat or metabolizes it only if energy intake changes for a period of several days. Once you have been on a major diet, the next one is less successful because your body alters the way it responds to low energy intake. Your basal metabolic rate is the rate at which food is converted into heat transfer and work done while the body is at complete rest. The body adjusts its basal metabolic rate to compensate (partially) for over-eating or under-eating. The body will decrease the metabolic rate rather than eliminate its own fat to replace lost food intake. You will become more easily chilled and feel less energetic as a result of the lower metabolic rate, and you will not lose weight as fast as before. Exercise helps with weight loss because it produces both heat transfer from your body and work, and raises your metabolic rate even when you are at rest.
Irreversibility
The body provides us with an excellent indication that many thermodynamic processes are irreversible. An irreversible process can go in one direction but not the reverse, under a given set of conditions. For example, although body fat can be converted to do work and produce heat transfer, work done on the body and heat transfer into it cannot be converted to body fat. Otherwise, we could skip lunch by sunning ourselves or by walking down stairs. Another example of an irreversible thermodynamic process is photosynthesis. This process is the intake of one form of energy—light—by plants and its conversion to chemical potential energy. Both applications of the first law of thermodynamics are illustrated in . One great advantage of such conservation laws is that they accurately describe the beginning and ending points of complex processes (such as metabolism and photosynthesis) without regard to the complications in between.