According to the first law of thermodynamics, heat transferred to a system can be either converted to internal energy or used to do work to the environment. A process in which a gas does work on its environment at constant pressure is called an isobaric process, while one in which volume is kept constant is called an isochoric process.
Isobaric Process (Constant Pressure)
An isobaric process occurs at constant pressure. Since the pressure is constant, the force exerted is constant and the work done is given as PΔV. An example would be to have a movable piston in a cylinder, so that the pressure inside the cylinder is always at atmospheric pressure, although it is isolated from the atmosphere. In other words, the system is dynamically connected, by a movable boundary, to a constant-pressure reservoir. If a gas is to expand at a constant pressure, heat should be transferred into the system at a certain rate. This process is called an isobaric expansion .
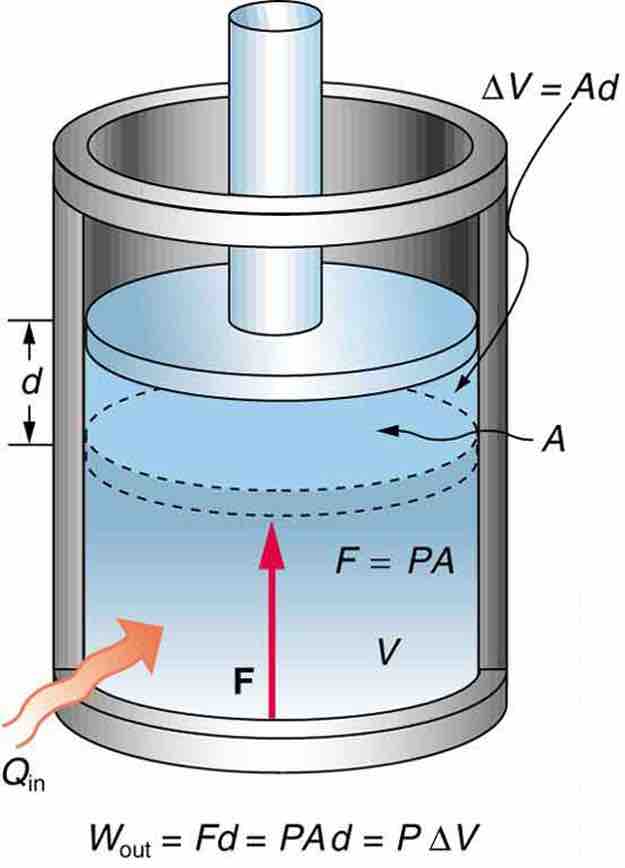
Fig 1
An isobaric expansion of a gas requires heat transfer during the expansion to keep the pressure constant. Since pressure is constant, the work done is PΔV.
Isochoric Process (Constant Volume)
An isochoric process is one in which the volume is held constant, meaning that the work done by the system will be zero. It follows that, for the simple system of two dimensions, any heat energy transferred to the system externally will be absorbed as internal energy. An isochoric process is also known as an isometric process or an isovolumetric process. An example would be to place a closed tin can containing only air into a fire. To a first approximation, the can will not expand, and the only change will be that the gas gains internal energy, as evidenced by its increase in temperature and pressure. Mathematically,
We may say that the system is dynamically insulated, by a rigid boundary, from the environment.