Concept
Version 9
Created by Boundless
Stastical Interpretation of Entropy
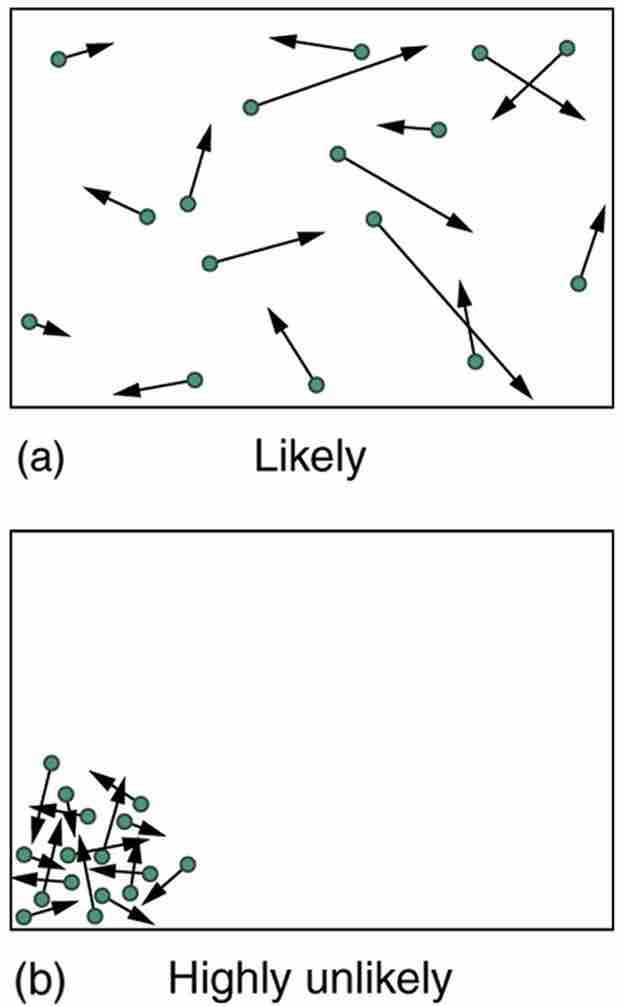
Kinetic Theory
(a) The ordinary state of gas in a container is a disorderly, random distribution of atoms or molecules with a Maxwell-Boltzmann distribution of speeds. It is so unlikely that these atoms or molecules would ever end up in one corner of the container that it might as well be impossible. (b) With energy transfer, the gas can be forced into one corner and its entropy greatly reduced. But left alone, it will spontaneously increase its entropy and return to the normal conditions, because they are immensely more likely.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, College Physics. February 13, 2013."
http://cnx.org/content/m42238/latest/?collection=col11406/latest
OpenStax CNX
CC BY 3.0.