Radiation is a natural process of heat transfer; everything is constantly radiating heat. When a system is at equilibrium, we do not notice this exchange of energy because every object absorbs and emits precisely the amount of energy it receives, but this equilibrium is dynamic--the exchange is always taking place. In general, as the temperature of an object increases, it emits energy more rapidly by emitting photons at a faster rate, and by emitting photons of a greater average energy.
If we approximate the Earth as a perfect absorber and emitter of the radiation received from the sun (called a blackbody), we would expect the Earth to be at an average temperature of 5°C, rather than the 14°C which we observe. The extra temperature comes from the Earth and it's atmosphere selectively absorbing certain wavelengths of radiation while reflecting other wavelengths. The 9°C discrepancy is due to the greenhouse effect. The gases of the atmosphere are "selective absorbers"; energy in the visible part of the electromagnetic spectrum passes through the atmosphere directly to the Earth's surface (with some reflection occurring as well). The Earth absorbs this energy and then re-emits radiation in the infrared portion of the spectrum. The gases in the atmosphere, primarily CO2 and water vapor are highly absorbent in the infrared part of the spectrum. The atmosphere absorbs the infrared radiation from the Earth, preventing it from escaping to space. Because all objects are continually emitting radiation, the atmosphere (having absorbed the Earth's radiation) then emits radiation, some of which is then reabsorbed by the Earth's surface. Thus the greenhouse effect is a continuous cycle of absorption and emission of energy between the Earth and atmosphere. This causes the Earth's atmosphere and surface to be warmer than otherwise expected.
Radiative Transfer
The greenhouse effect is a phenomenon of radiative transfer, the process by which the energy of light waves is exchanged in matter. Radiative transfer dictates what energy is reflected, absorbed, and emitted .
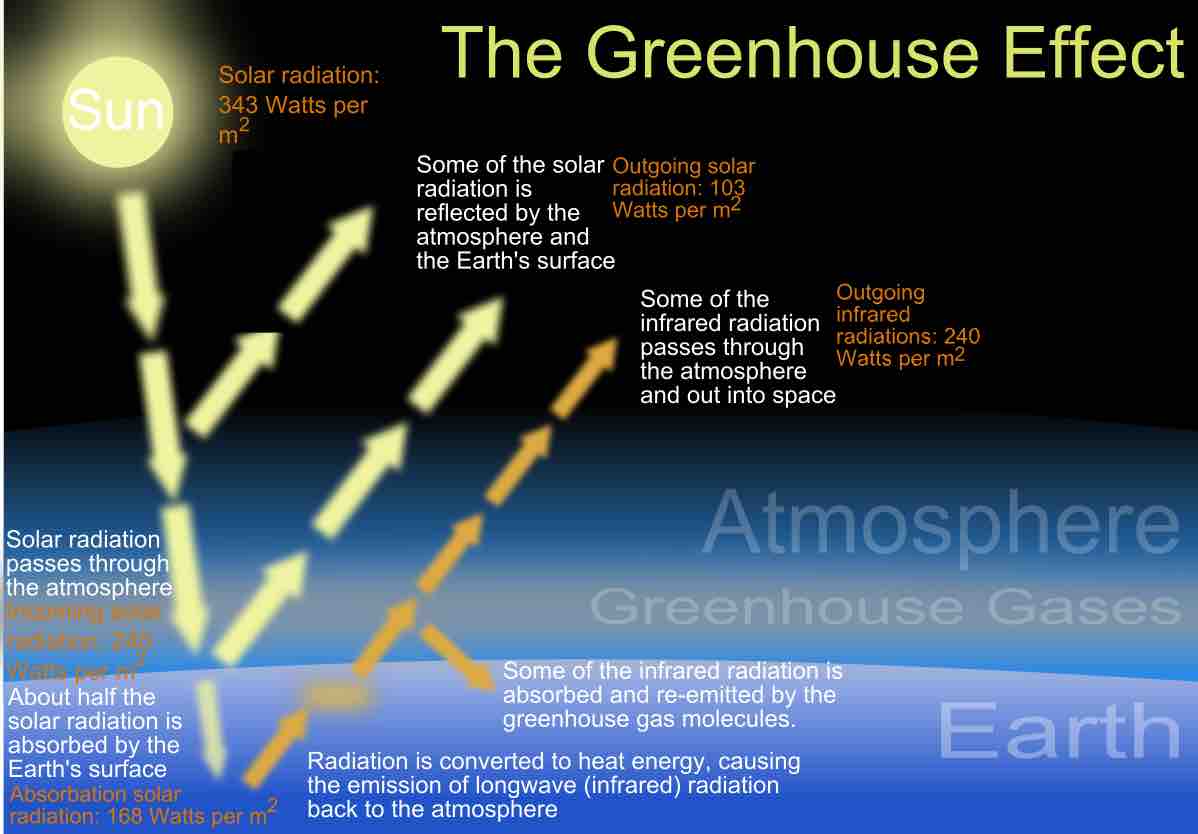
The greenhouse effect
A summary of the heat transfer in the Earth's atmosphere.
Atmospheric Absorbers
The radiative transfer properties of atmospheric chemicals depend on the energy of the radiation (both for absorption and emission), and those properties are unique to each chemical. In the context of global warming, we find that it is important to consider both how chemicals and particles reflect sunlight and how they absorb energy radiated from the Earth. Atmospheric reflecters, notably sulfates and nitrates, reflect and scatter light before it ever hits the surface of the Earth, effectively reducing the power that the Earth receives. On the other hand, greenhouse gases such as carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) are characteristically strong absorbers of the energy radiated by the Earth's surface. They absorb so strongly because they typically exhibit resonant absorption behavior in the same energy range as the radiation emitted by the earth. These trap heat before it leaves the Earth, insulating the Earth and increasing the Earth's equilibrium temperature.
Entropy and Solar Radiation
The Second Law of Thermodynamics implies that in order to produce energy sources, entropy must be produced. On a planetary scale, this production of entropy is primarily accounted for by the absorption and re-emisson of radiation. In general, the earth radiates the same energy that it receives. (If greenhouse gases increase, then temperature increases, and higher temperatures cause the Earth to radiate more, compensating for the greater energy absorbed in the atmosphere. ) With light radiation, we find the entropy carried by the photons decreases as temperature increases,