Overview of Atomic Electrical Charges
Atoms, the fundamental building blocks of all molecules, consist of three types of particles: protons, neutrons, and electrons. Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net charge.
Both protons and electrons have charge that is quantized. That is, the magnitude of their respective charges, which are equal each other, is 1. This standard value is equal to approximately 1.6×10-19 Coulombs.
Protons
Protons are found in the center of the atom; they, with neutrons, make up the nucleus. Protons have a charge of +1 and a mass of 1 atomic mass unit, which is approximately equal to 1.66×10-24 grams. The number of protons in an atom defines the identity of the element (an atom with 1 proton is hydrogen, for example, and an atom with two protons is helium). As such, protons are relatively stable; their number rarely changes, only in the instance of radioactive decay.
Electrons
Electrons are found in the periphery of the atom and have a charge of -1. They are much smaller than protons; their mass is
Ions
In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Atoms in such a state are known as ions.
If a neutral atom gains an electron, it becomes negative. This kind of ion is called an anion.
If a neutral atom loses an electron, it becomes positive. This kind of ion is called a cation.
The steady flow of electrons is called current. Current is what flows through electrical wires and powers electronics items, from light bulbs to televisions.
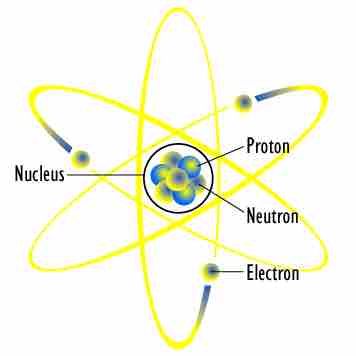
Planetary Model of an Atom
Small electrons orbit the large and relatively fixed nucleus of protons and neutrons.