Nineteen families of bacteriophages that infect bacteria and archaea are currently recognized. Of these, only two families have RNA genomes: Cystoviridae (segmented dsRNA) and Leviviridae (linear ssRNA).
The Leviviridae include the genera Allolevivirus (type species: Enterobacteria phage Qβ) and Levivirus (type species: Enterobacteria phage MS2).
Cystovirus is a genus of dsRNA virus that infect certain Gram-negative bacteria. All cystoviruses are distinguished by their three strands (analogous to chromosomes) of dsRNA, totalling ~14 kb in length, and by their protein and lipid outer layer . No other bacteriophage has any lipid in its outer coat, though the Tectiviridae and the Corticoviridae have lipids within their capsids.
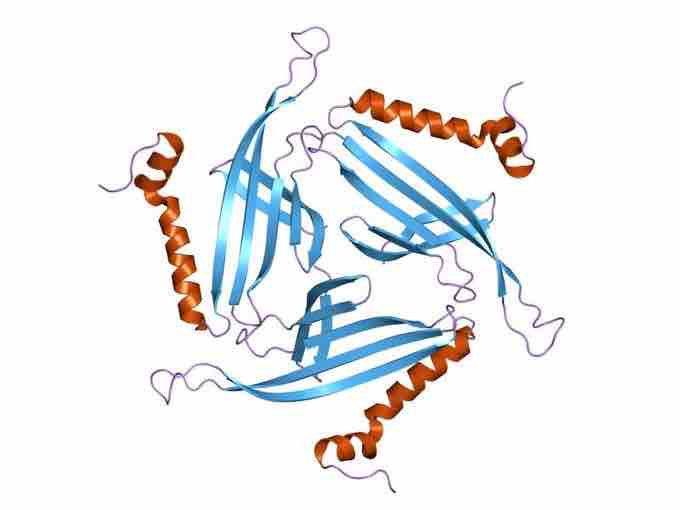
Structure of bacteriophage PP7 from Pseudomonas aeruginosa
Members of this protein family form the capsid of Pseudomonas phage PP7. They adopt a secondary structure consisting of a six-stranded beta sheet and an alpha helix.
Most identified cystoviruses infect Pseudomonas species, but this is likely biased due to the method of screening and enrichment. The type species is Pseudomonas phage Φ6, but there are many other members of this family: Φ7, Φ8, Φ9, Φ10, Φ11, Φ12, and Φ13 have been identified and named, but other cystoviruses have also been isolated.
Members of the Cystoviridae appear to be most closely related to the Reoviridae, but also share homology with the Totiviridae. Cystoviruses are the only bacteriophage that are more closely related to viruses of eukaryotes than to other phage.
Bacteriophage Φ6 is a member of the Cystoviridae family. It infects Pseudomonas bacteria (typically plant-pathogenic P. syringae). It has a three-part, segmented, double-stranded RNA genome, totalling ~13.5 kb in length. Φ6 and its relatives have a lipid membrane around their nucleocapsid, a rare trait among bacteriophages. It is a lytic phage, though under certain circumstances has been observed to display a delay in lysis that may be described as a "carrier state. "
Φ6 typically attaches to the Type IV pilus of P. syringae with its attachment protein, P3. It is thought that the cell then retracts its pilus, pulling the phage toward the bacterium. Fusion of the viral envelope with the bacterial outer membrane is facilitated by the phage protein, P6. The muralytic (peptidoglycan-digesting) enzyme, P5, then digests a portion of the cell wall, and the nucleocapsid enters the cell coated with the bacterial outer membrane.
RNA-dependent RNA polymerases (RdRPs) are critical components in the life cycle of double-stranded RNA (dsRNA) viruses. However, it is not fully understood how these important enzymes function during viral replication. Expression and characterization of the purified recombinant RdRP of Φ6 is the first direct demonstration of RdRP activity catalyzed by a single protein from a dsRNA virus. The recombinant Φ6 RdRP is highly active in vitro, possesses RNA replication and transcription activities, and is capable of using both homologous and heterologous RNA molecules as templates.