Herpes simplex viruses 1 and 2 (HSV-1 and HSV-2) are two members of the herpes virus family, Herpesviridae, that infect humans. Both HSV-1 (which produces most cold sores) and HSV-2 (which produces most genital herpes) are ubiquitous and contagious. They can be spread when an infected person is producing and shedding the virus.
The sequential stages of HSV entry are analogous to those of other viruses. At first, complementary receptors on the virus and the cell surface bring the viral and cell membranes into close proximity. In an intermediate state, the two membranes begin to merge, forming a hemifusion state. Finally, a stable entry pore is formed through which the viral envelope contents are introduced to the host cell .
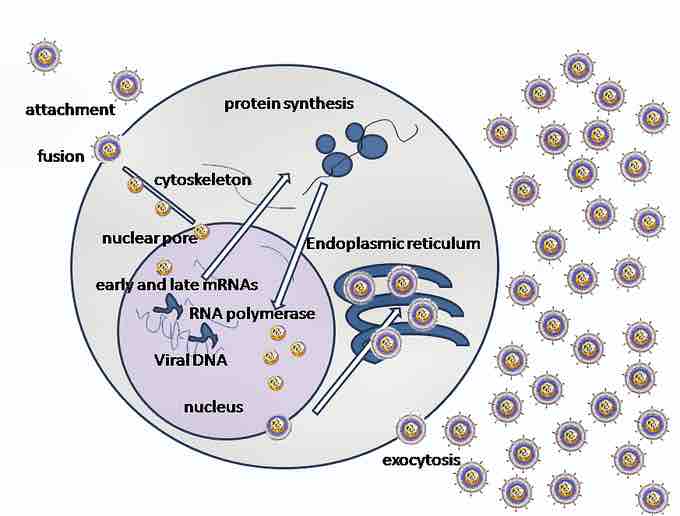
Virus replication
Herpes simplex virus attaches to host cell surface receptors using glycoproteins. Following attachment, the viral envelope fuses with the host cell membrane and the viral capsid gains entry into the cell.
The genome encodes for 11 different glycoproteins, four of which, gB, gC, gD and gH, are involved in viral attachment. Initial interactions occur when viral envelope glycoprotein C (gC) binds to a cell surface particle called heparan sulfate. A second glycoprotein, glycoprotein D (gD), binds specifically to at least one of three known entry receptors. These include herpesvirus entry mediator (HVEM), nectin-1 and 3-O sulfated heparan sulfate. The receptor provides a strong, fixed attachment to the host cell. These interactions bring the membrane surfaces into mutual proximity and allow for other glycoproteins embedded in the viral envelope to interact with other cell surface molecules. Once bound to the HVEM, gD changes its conformation and interacts with viral glycoproteins H (gH) and L (gL), which form a complex. The interaction of these membrane proteins results in the hemifusion state. Afterward, gB interaction with the gH/gL complex creates an entry pore for the viral capsid. Glycoprotein B interacts with glycosaminoglycans on the surface of the host cell.