Methylotrophs
Multiple diverse microorganisms have evolved the intriguing ability to utilize single-carbon (C1) compounds (e.g. methanol or methane) or multi-carbon compounds lacking carbon bonds (e.g. dimethyl ether and dimethylamine) as the sole energy source for their growth. Microbes with this capability are known as methylotrophs.
Methylotrophs, in general, aerobically utilize C1 compounds by oxidizing them to yield formaldehyde. Formaldehyde, in turn, can either be "burned" for energy (by dissimilation to CO2) or assimilated into biomass, allowing the cell to grow using molecules like methanol as a sole carbon source. Because methanol is more abundant, more easily purified, and cheaper than sugar carbon sources (e.g. glucose), methylotrophs are particularly useful in biotechnology for the production of amino acids, vitamins, recombinant proteins, single-cell proteins, co-enzymes, and cytochromes .
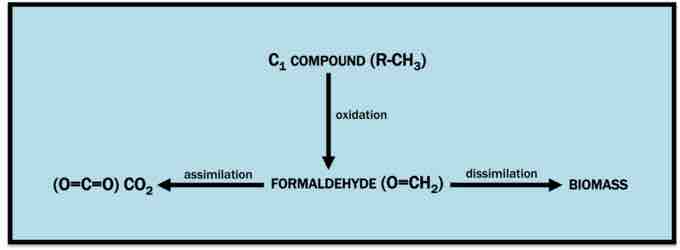
Methylotrophy
This is the general utilization pathway for C1 compounds. Molecules of this type are converted to formaldehyde by oxidation and ultimately used as a source of energy and carbon.
Here are examples of methylotrophs:
- Methanosarcina, which can both utilize and produce methane;
- Methylococcus capsulatus, which requires methane to survive; and
- Pichia pastoris, a biotechnologically important model organism that can use methanol as a carbon and energy source.
Methanotrophs
Some methylotrophs can degrade the greenhouse gas methane. Organisms of this type are referred to as methanotrophs. Methanotrophs are able to metabolize methane as their only source of carbon and energy. Most known methanotrophs are bacteria that strictly require methane for growth ("obligate methanotrophs"). The fact that some methylotrophs can also make use of multi-carbon compounds distinguishes them from methanotrophs, which are usually fastidious methane and methanol oxidizers.
Methanotrophs occur mostly in soils. They are especially common near environments where methane is produced, such as:
- oceans
- mud
- marshes
- underground environments
- soils
- rice paddies
- landfills
Methanotrophs are of special interest to researchers studying global warming because they prevent a potential greenhouse gas (methane), far more potent than carbon dioxide, from being released into the atmosphere. Methanophilic ("methane-loving") bacteria, therefore, are significant in the global methane budget.
Methane Utilization
Methanotrophs oxidize methane by first initiating reduction of oxygen (O2) to water (H2O) and oxidation of methane (CH4) to a more active species, methanol (CH3OH), using oxidoreductase enzymes called methane monooxygenases (MMOs). Two types of MMO have been isolated from methanotrophs:
- soluble methane monooxygenase (sMMO), which is found in the cell cytoplasm.
- particulate methane monooxygenase (pMMO), which is found in the cell membrane.
Cells containing pMMO demonstrate higher growth capabilities and higher affinity for methane than cells that contain sMMO. Because pMMO is a membrane protein, cells that use it for methane metabolism characteristically have a system of internal membranes within which methane oxidation occurs .
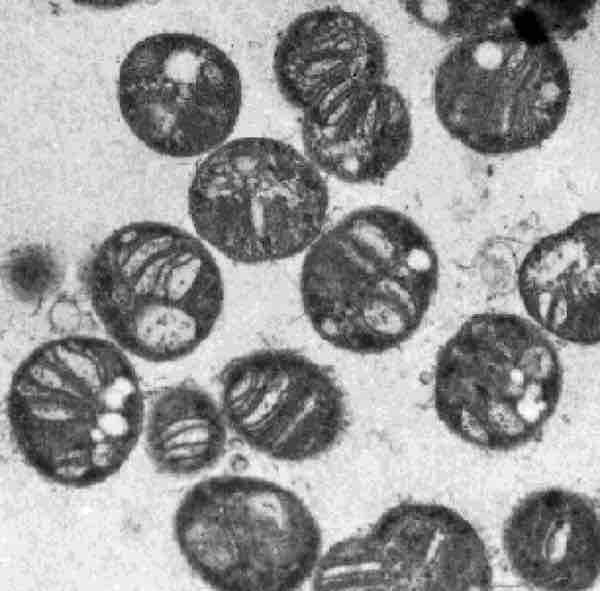
Methylococcus capsulatus
The Methylococcaceae have internal membranes (in the form of flattened discs) that contain pMMOs, which catalyze methane oxidation.
As in the general case described above for methylotrophs, methanotrophs ultimately oxidize the methanol produced by MMOs to yield formaldehyde. The method of formaldehyde fixation differs between various methanotrophic organisms. This difference (along with variability in membrane structure) divides methanotrophs into several subgroups, such as the Methylococcaceae, Methylocystaceae, and Verrucomicrobiae. Although the mechanism by which it occurs is not entirely clear, it is also apparent that certain bacteria can utilize methane anaerobically by essentially running the methanogenesis pathway (normally used by methanogenic bacteria to produce methane) in reverse. This typically occurs in microbes dwelling in marine sediments where oxygen is scarce or altogether absent.