Virtually all microbes can trigger an antibody response. Successful recognition and eradication of many different types of microbes requires diversity among antibodies (glycoproteins belonging to the immunoglobulin superfamily). It is the variety in their amino acid composition that allows them to interact with many different antigens. It has been estimated that humans generate about 10 billion different antibodies, each capable of binding a distinct epitope of an antigen. Although a huge repertoire of different antibodies is generated in a single individual, the number of genes available to make these proteins is limited by the size of the human genome. Several complex genetic mechanisms have evolved that allow vertebrate B cells to generate a diverse pool of antibodies from a relatively small number of antibody genes.
Antibody Structure
Antibodies are typically made of basic structural units—each with two large heavy chains and two small light chains . There are several different types of antibody heavy chains, and several different kinds of antibodies, which are grouped into different isotypes based on which heavy chain they possess. Five different antibody isotypes are known in mammals, which perform different roles, and help direct the appropriate immune response for each different type of foreign object they encounter. Though the general structure of all antibodies is very similar, a small region at the tip of the protein is extremely variable, allowing millions of antibodies with slightly different antigen binding sites to exist. This region is known as the hypervariable region. Each of these variants can bind to a different antigen. This enormous diversity of antibodies allows the immune system to recognize an equally wide variety of antigens.
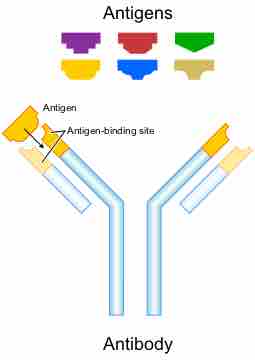
Antibodies bind to specific antigens
Schematic diagram of an antibody and antigens. Light chains are in lighter blue and orange, heavy chains in darker blue and orange. Each antibody binds to a specific antigen; an interaction similar to a lock and key.
Antibodies obtain their diversity through two processes:
V(D)J Recombination
The first stage is called somatic, or V(D)J, which stands for variable, diverse, and joining regions recombination. Several sets of genes are located within each of the three regions. During cell maturation, the B cell will splice out the DNA of all but one of the genes from each region and combine the three remaining genes together to form one VDJ segment. This segment, along with a constant region gene, forms the basis for subsequent antibody production.
It is estimated that given the number of variants in each of the three regions, approximately 10,000-20,000 unique antibodies are producible . V(D)J recombination takes place in the primary lymphoid tissue (bone marrow for B cells, and thymus for T cells) and nearly randomly combines variable, diverse, and joining gene segments. It is due to this randomness in choosing different genes that it is able to diversely encode proteins to match antigens.
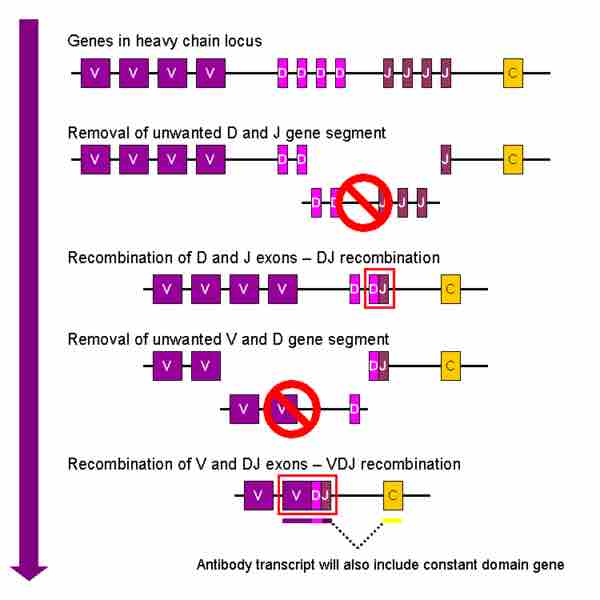
Redistribution within the immunoglobulin (antibody) gene
Schematic overview of V(D)J recombination.
Somatic Hypermutation
The second stage of recombination occurs after the B cell is activated by an antigen. In these rapidly dividing cells, the genes encoding the variable domains of the heavy and light chains undergo a high rate of point mutation, by a process called somatic hypermutation (SHM). SHM is a cellular mechanism by which the immune system adapts to the new foreign elements that confront it and is a major component of the process of affinity maturation. SHM diversifies B cell receptors used to recognize antigens and allows the immune system to adapt its response to new threats during the lifetime of an organism. Somatic hypermutation involves a programmed process of mutation affecting the variable regions of immunoglobulin genes. SHM results in approximately one nucleotide change per variable gene, per cell division. As a consequence, any daughter B cells will acquire slight amino acid differences in the variable domains of their antibody chains. This serves to increase the diversity of the antibody pool and impacts the antibody's antigen-binding affinity. Some point mutations will result in the production of antibodies that have a lower affinity with their antigen than the original antibody, and some mutations will generate antibodies with a higher affinity. B cells that express higher affinity antibodies on their surface will receive a strong survival signal during interactions with other cells, whereas those with lower affinity antibodies will not, and will die by apoptosis. Thus, B cells expressing antibodies with a higher affinity for the antigen will outcompete those with weaker affinities for function and survival. The process of generating antibodies with increased binding affinities is called affinity maturation. Affinity maturation occurs after V(D)J recombination, and is dependent on help from helper T cells.
Antibody genes also re-organize in a process called class switching, which changes the base of the heavy chain to another. This creates a different isotype of the antibody while retaining the antigen specific variable region, thus allowing a single antibody to be used by several different parts of the immune system.