Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central atom. Usually these ligands are organic compounds and are called chelants, chelators, chelating agents, or sequestering agents; the resulting complexes are called chelate compounds.
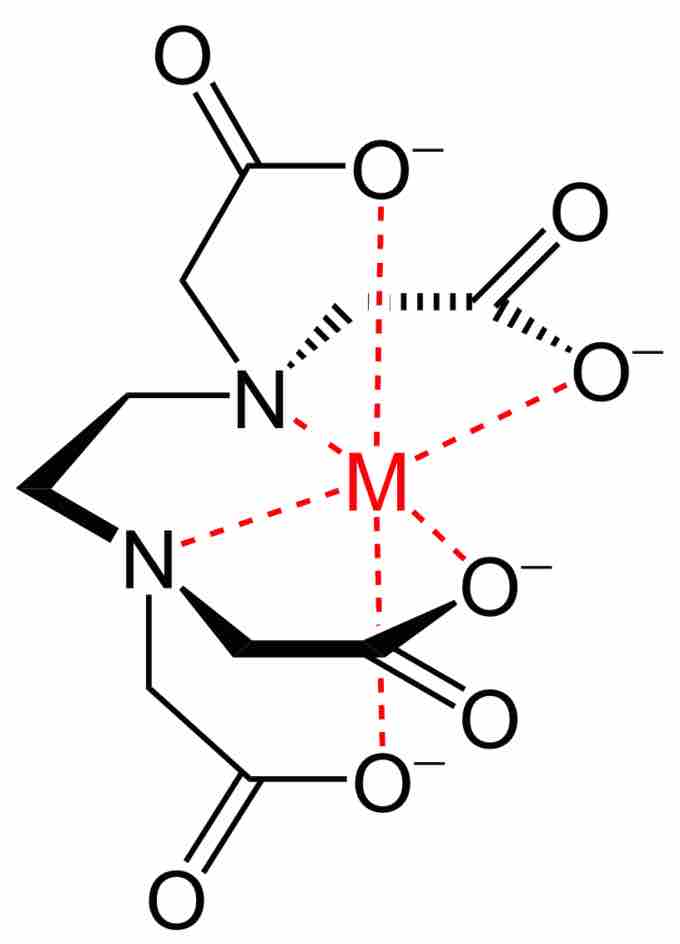
Metal-EDTA chelate
Chemical structure of EDTA chelate.
Chelate complexes are contrasted with coordination complexes composed of monodentate ligands, which form only one bond with the central atom. Chelating agents, unlike the other ligands in coordination compounds, bind via multiple atoms in the ligand molecule, not just one.
The Chelate Effect
The chelate effect describes the enhanced affinity of chelating ligands for a metal ion compared to the affinity of a collection of similar nonchelating (monodentate) ligands for the same metal. Consider the two equilibria, in aqueous solution, between the copper(II) ion (Cu2+) and ethylenediamine (en) on the one hand and methylamine (CH3NH2 (MeNH2)) on the other.
In (1), the bidentate ligand ethylenediamine forms a chelate complex with the copper ion. Chelation results in the formation of a five-membered ring. In (2), the two monodentate methylamine ligands of approximately the same donor power (the enthalpy of formation of Cu—N bonds is approximately the same in the two reactions) forms a complex. Under conditions of equal copper concentrations and when the concentration of methylamine is twice the concentration of ethylenediamine, the concentration of the complex in (1) will be greater than the concentration of the complex in (2). The effect increases with the number of chelate rings, so the concentration of the EDTA complex, which has six chelate rings, is much higher than a corresponding complex with two monodentate nitrogen donor ligands and four monodentate carboxylate ligands. Thus, the phenomenon of the chelate effect is a firmly established empirical fact.
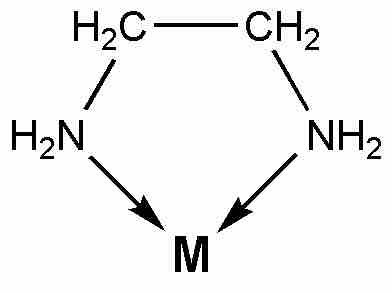
Ethylenediamine chelate
Ethylenediamine serves as a chelating agent by binding via its two nitrogen atoms.
Applications of Chelating Agents
Chelation therapy is the use of chelating agents to detoxify poisonous metal agents, such as mercury, arsenic, and lead, by converting them to a chemically inert form that can be excreted without further interaction with the body. This therapy was approved by the U.S. Food and Drug Administration in 1991. Although they can be beneficial in cases of heavy metal poisoning, chelating agents also can be dangerous. Use of disodium EDTA instead of calcium EDTA has resulted in fatalities due to hypocalcemia.
Virtually all biochemicals exhibit the ability to dissolve certain metal cations. Thus, proteins, polysaccharides, and polynucleic acids are excellent polydentate ligands for many metal ions. Organic compounds such as the amino acids glutamic acid and histidine, organic diacids such as malate, and polypeptides such as phytochelatin are also typical chelators. In addition to these adventitious chelators, several biomolecules are specifically produced to bind certain metals. Virtually all metalloenzymes feature metals that are chelated, usually to peptides or cofactors and prosthetic groups. Such chelating agents include the porphyrin rings in hemoglobin and chlorophyll. Many microbial species produce water-soluble pigments that serve as chelating agents, termed siderophores. For example, species of Pseudomonas are known to secrete pyocyanin and pyoverdin that bind iron. Enterobactin, produced by E. coli, is the strongest chelating agent known.