Oxidation Numbers
The oxidation number, or oxidation state, of an atom is the charge that would exist on the atom if the bonding were completely ionic. This oxidation number is an indicator of the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. Oxidation number are typically represented by small integers.
In simple ions, the oxidation number of the atom is the charge on the ion. For example, Na+, K+, and H+ all have oxidation numbers of +1. O2- and S2- have oxidation numbers of -2. In a molecule or compound, the oxidation number is the sum of the oxidation numbers of its constituent atoms.
Example: What is the oxidation number of C2H6 ?
Solution: The oxidation number of C is -3. The oxidation number of H is +1 (H+ has an oxidation number of +1). For the compound, we calculate the oxidation number as follows: 2(-3) + 6(+1) = 0. Therefore, the oxidation number of C2H6 is 0.
Many atoms, including most atoms with d subshells, can have several different oxidation numbers. In complex ions or molecules, the oxidation numbers of these atoms can be calculated if we assume that the oxidation numbers of the other atoms in the species are fixed.
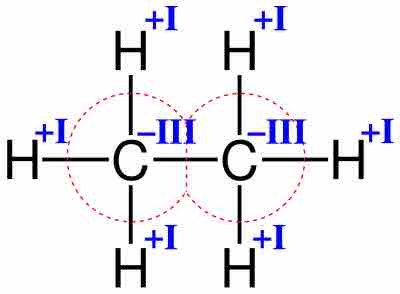
Oxidation number of ethane
Oxidation numbers of carbon and hydrogen in ethane.
Oxidation Numbers of Transition Metals
The oxidation number in coordination chemistry has a slightly different meaning. Transition metals are the elements in Groups 3 to 12 representing the d block of the periodic table. Due to the relatively low reactivity of unpaired d electrons, these metals typically form several oxidation states and therefore can have several oxidation numbers. Unfortunately, there is no simple rule to determining oxidation state possibilities among the transition metals, so it is best simply to memorize the common states of each element.
Example 1: An oxide of chromium has the formula Cr2O3. What is the oxidation number of each chromium ion?
Solution: The oxidation number of oxygen is assigned a charge of -2 when it reacts with a metal because the metal is more electropositive. The total charge of the oxygens is -6. Since the compound is neutral, the charge of each chromium must be +3 because 2(3)+3(-2) = 0.
Example 2: Another compound has the formula K2CrO4. What is the oxidation number of chromium?
Solution: The oxidation number for oxygen is assigned a charge of -2 when it reacts with a metal. Because there are 4 oxygen atoms, the total charge of the oxygens is -8. Potassium has an oxidation number of +1, giving an overall charge of +2. Because the compound is neutral and 2(1)+(Cr)+4(-2)=0, chromium must have an oxidation number of +6.
Multiple Oxidation States
The oxidation number for metals that can have more than one oxidation state is represented by a Roman numeral. The oxidation number is placed in parentheses after the name of the element (iron(III)). Sometimes, the oxidation states can also be written as a superscripted number to the right of the element symbol (Fe3+).
The maximum oxidation number in the first row of transition metals is equal to the number of valence electrons from scandium (+3) up to manganese (+7). However, it decreases in the latter elements. In the second and third rows, the maximum oxidation number is that of ruthenium and osmium (+8). In compounds such as (MnO4)− and OsO4, the elements achieve a stable octet by forming four covalent bonds.
Oxidation Numbers of Main-Group Elements
Main-group elements, those in Groups 13 to 17, also exhibit multiple oxidation states. The "common" oxidation states of these elements typically differ by two. For example, in compounds containing gallium the oxidation states of gallium are +1 and +3. No compound of gallium(II) is known; any such compound would have an unpaired electron and would behave as a free radical and be destroyed rapidly. The only compounds in which gallium has a formal oxidation state of +2 are dimeric compounds, such as [Ga2Cl6]2−, which contain a Ga-Ga bond formed from the unpaired electron on each Ga atom.