There are a few ways to approach this particular topic, and they all refer to how the elements on the table itself are presented.
The most classic representation of the periodic table shows the relative positions of the known elements in the table. The table itself is comprised of 7 periods and 18 groups, with the latest known element being number 118, ununoctium. However, there is a glaring discontinuity apparent in the table. In Row 6, Column 3, an empty space appears between Ba and Hf. The atomic number that should be here, 57, is located at the bottom of the table in the row called the Lanthanides. Directly below the space in Row 6, in Row 7, is another empty space, which is filled by a row called the Actinides, also seen at the bottom of the chart.
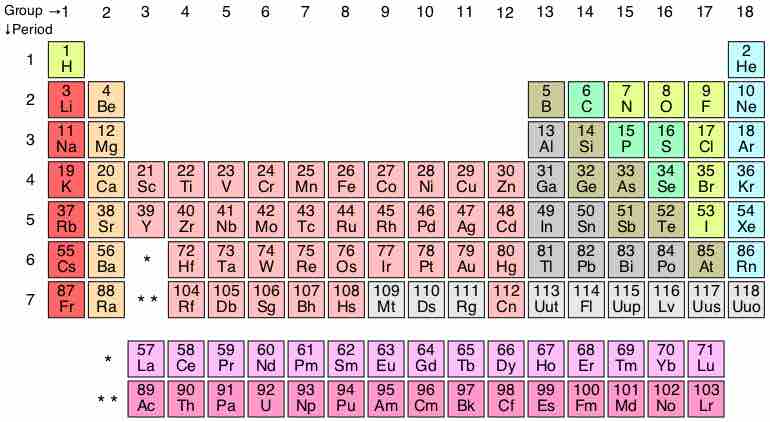
The periodic table
This is a standard representation of the elements in the table, with relative positions that are familiar to chemists and physicists.
Expanding the Dimensions of the Periodic Table
By expanding the horizontal dimensions of the table, the actinide and lanthanide rows can be put into their correct relative positions. Since the chemistries of this group rest largely on the f-shell electrons and the interactions at this energy level, this is called the f-block. This representation, clumsy as it is, correctly shows the elements known to date, up to z=118, unonoctium. In fact, this representation is predictive in that it shows chemical families (groups) and the periodicities (periods) in their correct relative positions.
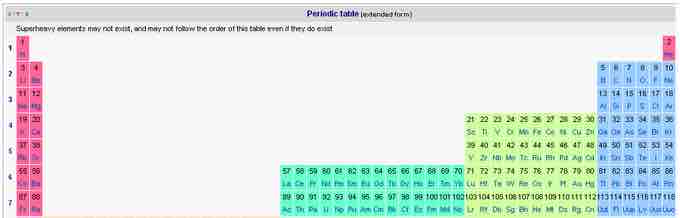
The extended periodic table
The lanthanides and actinides are added as separate but connected rows, building what is called the f-block.
Taking the extension of the periodic table even further, consider an element with atomic number 92 in the actinide series, called uranium. Once elements of this atomic number range were discovered at the end of the 19th century, isotopes of uranium were the largest and heaviest elements known in nature. In 1934, Enrico Fermi predicted the existence of transuranium elements—those elements with atomic number (z) greater than or equal to 93. In 1934 only 4 actinides were known, all smaller than uranium, so it was not known that they formed a period or family like the lanthanides. The first transuranium element, Np (neptunium), was synthetically produced in 1940 by bombarding uranium with slow neutrons. Over the next two decades, a great many actinide isotopes were produced, generally by bombardment with either other atoms or subatomic particles. The actinides were added along with the lanthanides.
Two New Periods
By using the predictive properties of the periodic table, along with a growing expertise in atomic and subatomic theory, two entirely new periods were predicted. On the advice of Glenn Seaborg and others, Periods 8 and 9 were added to the periodic table, comprising the g-block. The positioning of the g-block in the table (to the left of the f-block, to the right, or in between) is speculative. The positions in the table correspond to the assumption that the Madelung rule (that orbitals with lower value of the sum of n and l quantum numbers will be filled before those of higher n+l values) will continue to hold for higher atomic numbers. At element 118, the orbitals 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d, 5f, 6s, 6p, 6d, 7s, and 7p are assumed to be filled, with the remaining orbitals unfilled. The orbitals of the eighth period are predicted to be filled in the order 8s, 5g, 6f, 7d, 8p. However, after approximately element 120, the proximity of the electron shells makes placement in a simple table problematic.
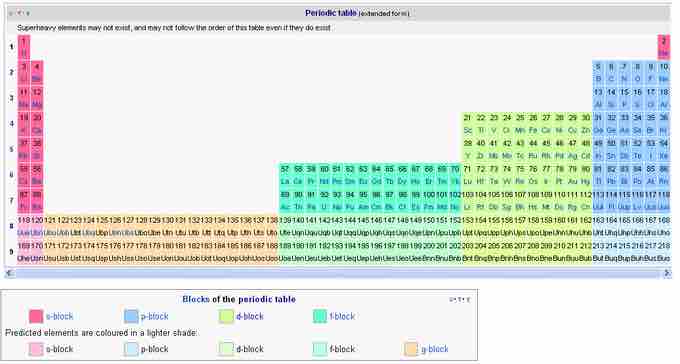
The extended periodic table with predicted Periods 8 and 9
The periodic table with all groups and periods in place. The placement of the undiscovered g-block is speculative.
The existence of elements with these high atomic numbers is speculative, and isotopes are expected to have fleetingly short half-lives. Various experts predict that z = approximately 130 is a maximum, while others feel that there is no effective upper limit. Experiments in the synthesis of transuranium elements continue.