Major Divisions of the Periodic Table
The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. Elements are presented in increasing atomic number. The main body of the table is a 18 × 7 grid. Elements with the same number of valence electrons are kept together in groups, such as the halogens and the noble gases. There are four distinct rectangular areas or blocks. The f-block is usually not included in the main table, but rather is floated below, as an inline f-block would often make the table impractically wide. Using periodic trends, the periodic table can help predict the properties of various elements and the relations between properties. It therefore provides a useful framework for analyzing chemical behavior and is widely used in chemistry and other sciences.
Atomic Orbitals
The electrons in the partially filled outermost shell (or shells) determine the chemical properties of the atom; it is called the valence shell. Each shell consists of one or more subshells, and each subshell consists of one or more atomic orbitals.
The properties of an atom depend ultimately on the number of electrons in the various orbitals, and on the nuclear charge which determines the compactness of the orbitals. In order to relate the properties of the elements to their locations in the periodic table, it is often convenient to make use of a simplified view of the atom in which the nucleus is surrounded by one or more concentric spherical "shells," each of which consists of the highest-principal quantum number orbitals that contain at least one electron; these are s- and p-orbitals and can include d- or f-orbitals, which is atom dependent. The shell model, as with any scientific model, is less a description of the world than a simplified way of looking at it that helps us to understand and correlate diverse phenomena.
We will look at several visualizations of the periodic table. First, however, it would be instructive to see how it is constructed from a logical viewpoint. The table today is the result of an ongoing effort of more than 100 years of observation, measurement, prediction and proof of the relationships of chemical and physical phenomena to electron configurations and charges.
Periods 1, 2, & 3
Starting with simple elements, the first three rows of the periodic table, called Periods 1, 2 and 3, correspond to the n=1, n=2 and n=3 levels.
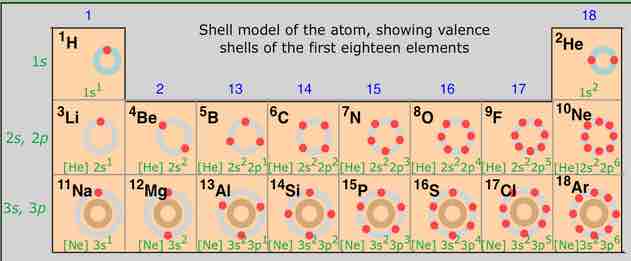
Electron shell configurations of the first 18 elements
The electron shell configurations of the first 18 elements in the periodic table. The corresponding energy levels (n) are listed in green numbers to the left. The number of outer-shell electrons is represented by the right-most digit in the group numbers.
Hydrogen has 1 electron in the 1s level, and to the right, helium, in Group 18, has 2 electrons in the 1s level, a completely filled shell, the duet rule. Helium is the first in the series of noble gases. Moving down to Period 2, lithium is the first element in the row, with a filled 1s configuration. Across the period, first the 2s and then the 2p orbitals fill, arriving at the configuration for neon, following the octet rule. Period 3 follows a similar pattern. Please note that the number of outer-shell electrons is the major determinant of the element's valence.
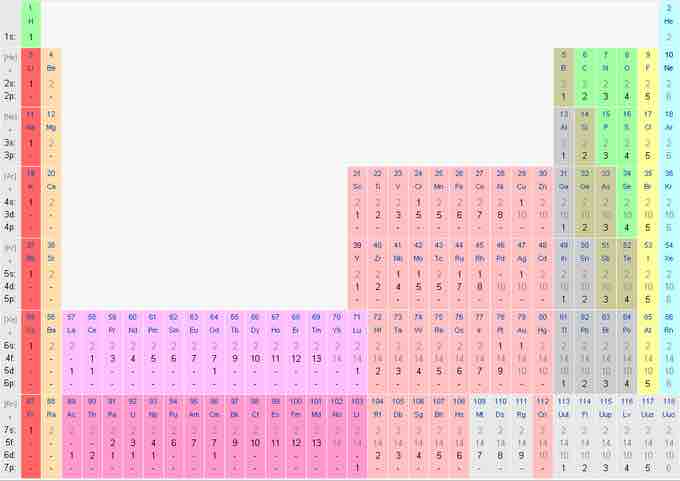
Electron shell configurations of the elements
Position in the periodic table based on electron shell configuration. This image shows the entire periodic table, with diagrammatic atoms and electron shells filling with movement through the table. This image breaks out the electron configuration numerically, showing the population of electrons in each subshell, starting each period with a completely filled noble gas.
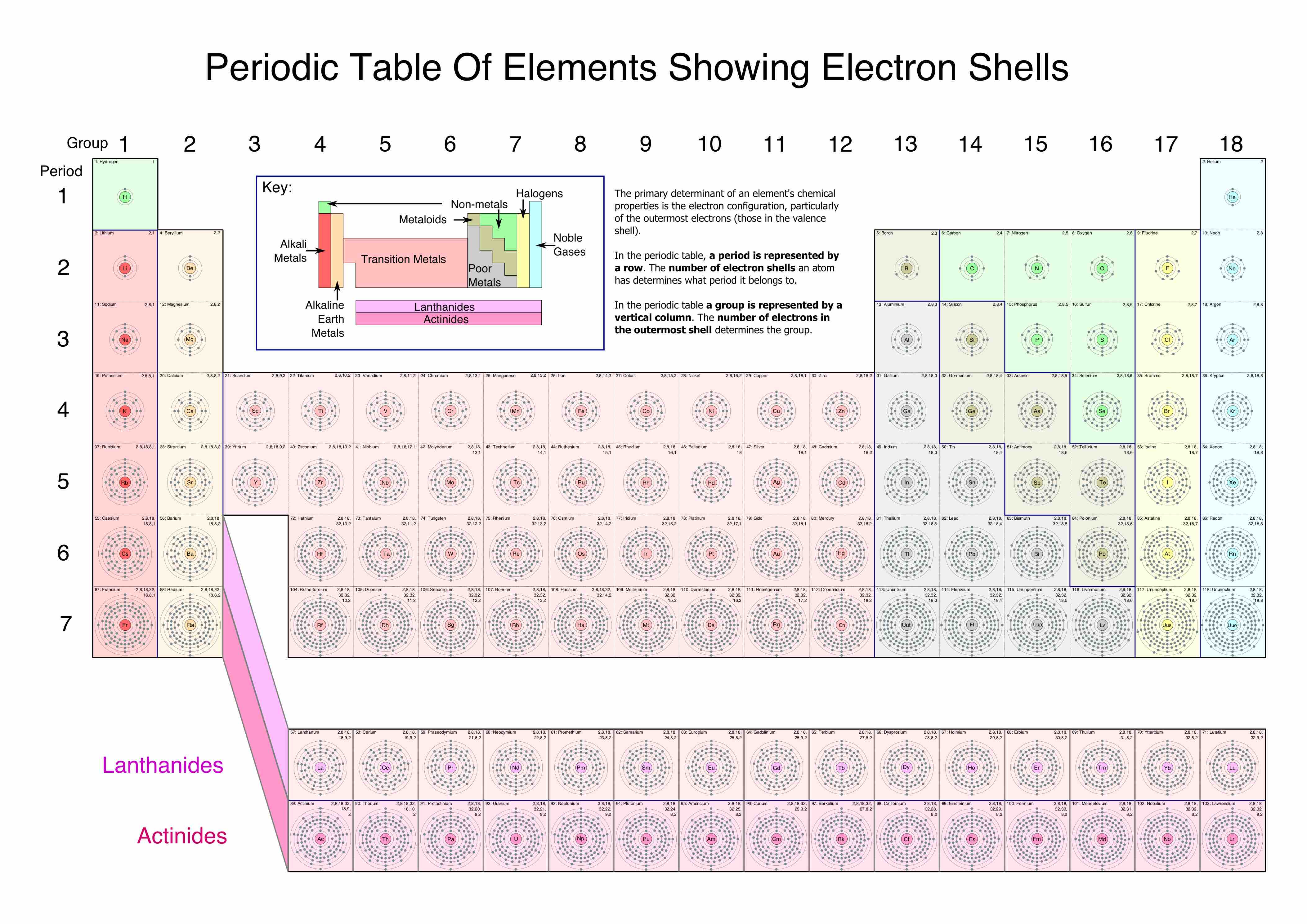
The periodic table showing electron shells
The elements in this table are laid out in the standard configuration of periods and groups. Each box includes representations of the electron shell structure for the element.