In chemistry, transuranium elements, also known as transuranic elements, are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. None of these elements is stable and each of them decays radioactively into other elements.
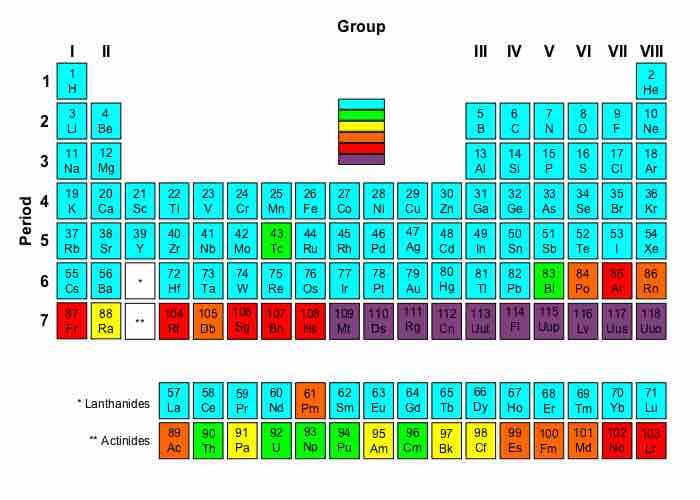
Periodic table radioactivity
Blue - Elements that contain at least one stable isotope. Green - Radioactive elements: the most stable isotope is very long-lived, with s half-life of over four million years. Yellow - Radioactive elements: the most stable isotope has a half-life between 800 and 34.000 years. Orange - Radioactive elements: the most stable isotope has a half-life between one day and 103 years. Red - Highly radioactive elements: the most stable isotope has a half-life between several minutes and one day. Purple - Extremely radioactive elements: the most stable isotope has a half-life less than several minutes. Very little is known about these elements due to their extreme instability and radioactivity.
Transuranium Elements in Nature
All of the elements with atomic numbers 1 to 92 can be found in nature and have stable or very long half-life isotopes. They can also be created as common products of the decay of uranium and thorium.
However, all of the elements with higher atomic numbers have been first discovered in the laboratory. Each of these elements is radioactive, with a half-life much shorter than the age of the Earth. So, if any atoms of these elements were ever present at the Earth's formation, they have long since decayed.
Producing Transuranium Elements
Transuranium elements that can be found on Earth now are artificially-generated, synthetic elements made via nuclear reactors or particle accelerators. The half-lives of these elements show a general trend of decreasing as atomic numbers increase. However, there are exceptions, including dubnium and several isotopes of curium. Further anomalous elements in this series have been predicted by Glenn T. Seaborg. They are categorized as the "island of stability."
Heavy transuranic elements are difficult and expensive to produce. Their prices go up rapidly with atomic number. As of 2008, weapons-grade plutonium cost around $4,000 per gram and californium cost $60,000,000 per gram. Due to production difficulties, none of the elements beyond californium has industrial applications, and of them, only einsteinium has ever been produced in macroscopic quantities.