Reviewing Fusion
Fusion power is the power generated by nuclear fusion processes. In fusion reactions, two light atomic nuclei fuse together to form a heavier nucleus. In doing so, they release a comparatively large amount of energy that arises from the binding energy, creating an increase in temperature of the reactants. To harness fusion power, a fusion reactor must be built to turn the energy released by fusion into electricity.
Harnessing Fusion Power
The term "fusion power" is commonly used to refer to potential commercial production of net usable power from a fusion source, similar to the usage of the term "steam power." Heat from the fusion reactions is used to operate a steam turbine which in turn drives electrical generators. This is similar to the process used in fossil fuel and nuclear fission power stations.
Conflicting Energies
The basic concept behind any fusion reaction is to bring two or more nuclei close enough so that the residual strong force (nuclear force) in their nuclei will pull them together into one larger nucleus. Fusion between the nuclei is opposed by the repulsive positive electrical charge common to all nuclei because they contain protons. To overcome this electrostatic force, or "Coulomb barrier," the kinetic energy of the atoms must be increased. The easiest way to do this is to heat the atoms, which has the side effect of stripping their electrons and leaving them as bare nuclei.
Fusion and Energy Concerns
If two light nuclei fuse, they will generally form a single nucleus with a slightly smaller mass than the sum of their original masses; this is not true in every case, though. The difference in mass is released as energy according to Albert Einstein's mass-energy equivalence formula, E = mc2. If the input nuclei are sufficiently massive, the resulting fusion product will be heavier than the sum of the reactants' original masses, in which case the reaction requires an external source of energy. The dividing line between "light" and "heavy" is iron-56. Above this atomic mass, energy will generally be released by nuclear fission reactions; below this mass, energy will be released by fusion.
In most experiments, the nuclei and electrons are left in a fluid known as a plasma, which is a state of matter that occurs when a gas is heated to extreme temperatures. The temperatures required to provide the nuclei with enough energy to overcome their repulsion is a function of the total charge. Therefore, hydrogen, which has the smallest nuclear charge, fuses at the lowest temperature, and is often used as fuel. Helium has an extremely low mass per nucleon and therefore is energetically favored as a fusion product. As a consequence, most fusion reactions combine isotopes of hydrogen (protium, 1H; deuterium, 2H or D; and tritium, 3H or T) to form isotopes of helium (3He or 4He) as the fusion end product.
Fuel cycles
The choice of fuel for a fusion reactor is dictated by a term called the Lawson criterion. It was first derived for fusion reactors by John D. Lawson in 1955, and published in 1957. The Lawson criterion is an important general measure of a system that defines the conditions needed for a fusion reactor to reach ignition. Namely, the heating of the plasma by the products of the fusion reactions must be sufficient to maintain the temperature of the plasma against all losses without external power input.
According to the Lawson criterion, the easiest and most immediately promising nuclear reaction for fusion power is:
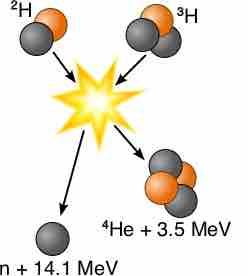
Fusion of deuterium with tritium
When 2-H and 3-H are fused, they produce 4-He, a neutron, and 14.1 MeV of energy, due to the conversion of mass into energy. This is because the rest of mass of helium and a neutron combined is less than the rest mass of deuterium and tritium combined, providing energy according to E=mc2.
Hydrogen-2, called deuterium, is a naturally occurring isotope of hydrogen and is commonly available. The large mass ratio of the hydrogen isotopes makes their separation easy compared to the difficult uranium enrichment process. Hydrogen-3, called tritium, is also an isotope of hydrogen, but it occurs naturally in only negligible amounts due to its half-life of 12.32 years. Consequently, the deuterium-tritium fuel cycle requires the breeding of tritium from lithium using one of the following reactions:
The reactant neutron is supplied by the D-T fusion reaction shown earlier. It is the one that has the greatest yield of energy. The reaction with 6Li is exothermic, providing a small energy gain for the reactor. The reaction with 7Li is endothermic but does not consume the neutron. At least some 7Li reactions are required to replace the neutrons lost to absorption by other elements.