Atomic Bombs
Atomic bombs are nuclear weapons that use the energetic output of nuclear fission to produce massive explosions. These bombs are in contrast to hydrogen bombs, which use both fission and fusion to power their greater explosive potential.
History
Only two nuclear weapons have been used in the course of warfare, both by the United States near the end of World War II. On August 6th, 1945, a uranium gun-type fission bomb code-named "Little Boy" was detonated over the Japanese city of Hiroshima. Three days later, on August 9th, a plutonium implosion-type fission bomb code-named "Fat Man" was exploded over Nagasaki, Japan. These two bombings resulted in the deaths of approximately 200,000 Japanese people—mostly civilians. The role of the bombings in Japan's surrender, and their ethical status, remain the subject of scholarly and popular debate.
Nuclear Chemistry Behind the Explosion
Atomic bombs are made up of a fissile element, such as uranium, that is enriched in the isotope that can sustain a fission nuclear chain reaction. When a free neutron hits the nucleus of a fissile atom like uranium-235 (235U), the uranium splits into two smaller atoms called fission fragments, plus more neutrons. Fission can be self-sustaining because it produces more neutrons with the speed required to cause new fissions. This creates the chain reaction.
The uranium-235 content of "weapons-grade" uranium is generally greater than 85 percent, though inefficient weapons, deemed "weapons-usable," can be made of 20 percent enriched uranium. The very first uranium bomb, Little Boy, dropped on Hiroshima in 1945, used 64 kilograms of 80 percent enriched uranium.
In fission weapons, a mass of fissile material, either enriched uranium or plutonium, is assembled into a supercritical mass—the amount of material needed to start an exponentially growing nuclear chain reaction. This is accomplished either by shooting one piece of sub-critical material into another, termed the "gun" method, or by compressing a sub-critical sphere of material using chemical explosives to many times its original density, called the "implosion" method.
The implosion method is considered more sophisticated than the gun method and only can be used if the fissile material is plutonium. The inherent radioactivity of uranium will then release a neutron, which will bombard another atom of 235U to produce the unstable uranium-236, which undergoes fission, releases further neutrons, and continues the process.The uranium atom can split any one of dozens of different ways, as long as the atomic weights add up to 236 (uranium plus the extra neutron). The following equation shows one possible split, namely into strontium-95 (95Sr), xenon-139 (139Xe), and two neutrons (n), plus energy:
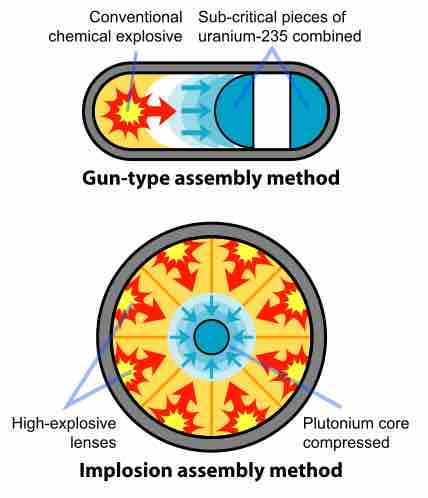
Fission bomb assembly methods
Two methods have been applied to induce the nuclear chain reaction that produces the explosion of an atomic bomb. The gun-type assembly uses a conventional explosive to compress from one side, while the implosion assembly compresses from all sides simultaneously.
The immediate energy release per atom is about 180 million electron volts (Me). Of the energy produced, 93 percent is the kinetic energy of the charged fission fragments flying away from each other, mutually repelled by the positive charge of their protons. This initial kinetic energy imparts an initial speed of about 12,000 kilometers per second.
However, the charged fragments' high electric charge causes many inelastic collisions with nearby nuclei, and thus these fragments remain trapped inside the bomb's uranium pit. Here, their motion is converted into X-ray heat, a process which takes about a millionth of a second. By this time, the material in the core and tamper of the bomb is several meters in diameter and has been converted to plasma at a temperature of tens of millions of degrees. This X-ray energy produces the blast and fire which are normally the purpose of a nuclear explosion.