Phosphorus is a chemical element with symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks. Elemental phosphorus exists in two major forms -- white phosphorus and red phosphorus -- but due to its high reactivity, phosphorus is never found as a free element on Earth.
While the term "phosphorescence" is derived from the ability of white phosphorus to glow faintly upon exposure to oxygen, the current chemical understanding is that this phenomenon is actually chemiluminescence, a mechanism of light emission distinct from phosphorescence.
Importance of Phosphorus
Phosphorus is essential for life. As evidence of the link between phosphorus and terrestrial life, elemental phosphorus was historically first isolated from human urine, and bone ash was an important early phosphate source. As phosphate, it is a component of DNA, RNA, ATP (adenosine triphosphate), and the phospholipids that form all cell membranes. Low phosphate levels are an important limit to growth in some aquatic systems, and the chief commercial use of phosphorus compounds for production of fertilizers is due to the need to replace the phosphorus that plants remove from the soil.
Phosphorus exists in several forms (allotropes) that exhibit strikingly different properties.
- The two most common allotropes are white phosphorus and red phosphorus.
- Another form, scarlet phosphorus, is obtained by allowing a solution of white phosphorus in carbon disulfide to evaporate in sunlight.
- Black phosphorus is obtained by heating white phosphorus under high pressures (about 12,000 standard atmospheres, or 1.2 gigapascals). In appearance, properties, and structure, black phosphorus resembles graphite -- it is black and flaky, a conductor of electricity, and has puckered sheets of linked atoms.
- Another allotrope is diphosphorus; it contains a phosphorus dimer as a structural unit and is highly reactive.
White Phosphorus and Related Molecular Forms
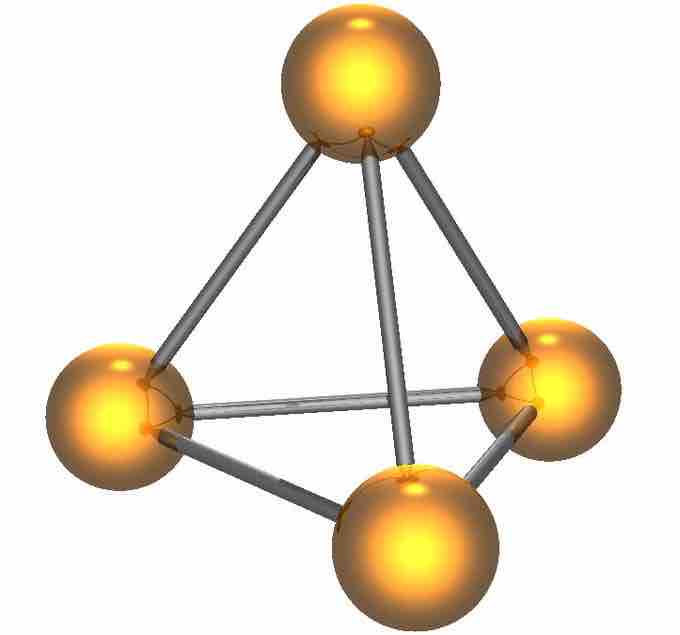
Diagram of White Phosphorus
The most important elemental form of phosphorus is white phosphorus, P4, which exhibits the bonding shown.
The most important elemental form of phosphorus in terms of applications is white phosphorus. It consists of tetrahedral P4 molecules, in which each atom is bound to the other three atoms by a single bond. This P4 tetrahedron is also present in liquid and gaseous phosphorus up to the temperature of 800 °C, at which point it starts decomposing into P2 molecules. Solid white phosphorus exists in two forms; at low temperatures, the β form is stable, and at high temperatures, the α form is predominant. These forms differ in terms of the relative orientations of the constituent P4 tetrahedra.

White Phosphorus
White phosphorus must be stored in an inert medium away from oxygen, such as in mineral oil, as shown here.
White phosphorus is the least stable, the most reactive, the most volatile, the least dense, and the most toxic of the allotropes. It gradually changes to red phosphorus, a transformation accelerated by light and heat. Samples of white phosphorus almost always contain some red phosphorus and so appear yellow. For this reason it is also called yellow phosphorus. It glows in the dark (when exposed to oxygen) with a very faint tinge of green and blue, and it is highly flammable and pyrophoric (self-igniting) upon contact with air. It is also toxic, causing severe liver damage upon ingestion. Owing to its pyrophoricity, white phosphorus is used as an additive in napalm. The odor of combustion of this form has a characteristic garlic smell, and samples are commonly coated with white "(di)phosphorus pentoxide," which consists of P4O10 tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is insoluble in water but soluble in carbon disulfide.
Red Phosphorus
Red phosphorus is polymeric in structure. It can be viewed as a derivative of P4 -- one of the P-P bonds is broken, and one additional bond is formed between the neighboring tetrahedrons, resulting in a chain-like structure. Red phosphorus may be formed by heating white phosphorus to 250 °C (482 °F) or by exposing it to sunlight. Phosphorus after this treatment is amorphous. Upon further heating, this material crystallizes. In this sense, red phosphorus is not an allotrope, but rather an intermediate phase between white and violet phosphorus, and most of its properties have a range of values. For example, freshly prepared, bright-red phosphorus is highly reactive and ignites at about 300 °C, though it is still more stable than white phosphorus, which ignites at about 30 °C. After prolonged heating or storage, the color darkens; the resulting product is more stable and does not spontaneously ignite in air.
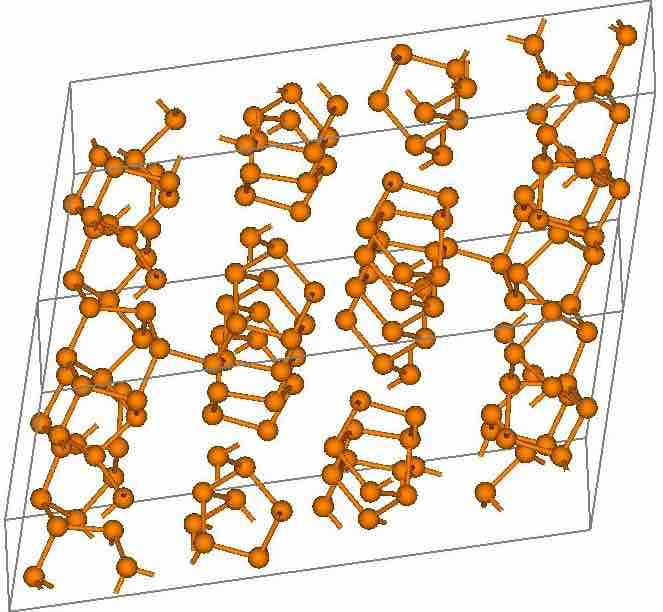
Red Phosphorus Crystal Structure
Red phosphorus is similar to P4 but is polymeric: one of the P-P bonds has been broken and is now attached to the next P4 unit.
Phosphorus Production
About 1,000,000 short tons (910,000 t) of elemental phosphorus is produced annually. Calcium phosphate (phosphate rock), mostly mined in Florida and North Africa, can be heated to 1,200-1,500 °C with sand, which is mostly SiO2, and coke (impure carbon) to produce vaporized P4. The product is subsequently condensed into a white powder underwater to prevent oxidation by air.