Properties of Carbon Oxides
Carbon oxides, or oxocarbons, are a class of organic compounds containing only carbon and oxygen. The most basic oxocarbons are carbon monoxide and carbon dioxide. Many other stable and metastable oxides of carbon are known but are rarely encountered.
Carbon Monoxide
The simplest oxocarbon is carbon monoxide (CO). Carbon monoxide is a colorless, tasteless gas that is slightly lighter than air. It is toxic to humans and animals when encountered in higher concentrations, despite the fact that it is produced in the metabolism and is thought to have some biological functions.
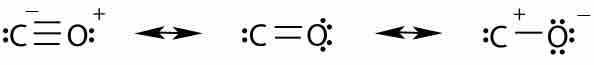
Carbon Monoxide
Carbon monoxide is stabilized by three different resonance structures. The first resonance structure is the most important one.
Carbon monoxide consists of one carbon and one oxygen atom connected by a triple bond. The distance between the carbon and oxygen atom is 112.8 pm, consistent with the presence of a triple bond. The bond dissociation energy of CO is 1072 kJ/mol and represents the strongest chemical bond known. CO has three resonance structures, but the structure with the triple bond is the best approximation of the real distribution of electron density in the molecule.
CO is naturally produced by the human body as a signaling molecule. Abnormalities in its metabolism have been linked to a variety of diseases, including hypertension and heart failure. CO is present in small amounts in the atmosphere, mostly as a result of the burning of fossil fuels and fires. Through natural processes in the atmosphere, it is eventually oxidized to carbon dioxide (CO2).
Carbon Dioxide
Carbon dioxide, or CO2, is a naturally occurring linear compound composed of two oxygen atoms covalently bonded to a carbon atom. The two C=O bonds are equivalent and short (116.3 pm), consistent with double bonding. The compound is centrosymmetric and so has no net dipole. CO2 is colorless; at high concentrations it has a sharp, acidic odor, but at lower concentrations it is odorless. At standard temperature and pressure, its density is 1.98 kg/m3, about 1.5 times that of air. It has no liquid state at pressures below 520 kPa; at 1 atm, the gas deposits directly to a solid at temperatures below -78.5 °C, and the solid sublimes directly to gas above this temperature. Solid CO2 is known as dry ice.
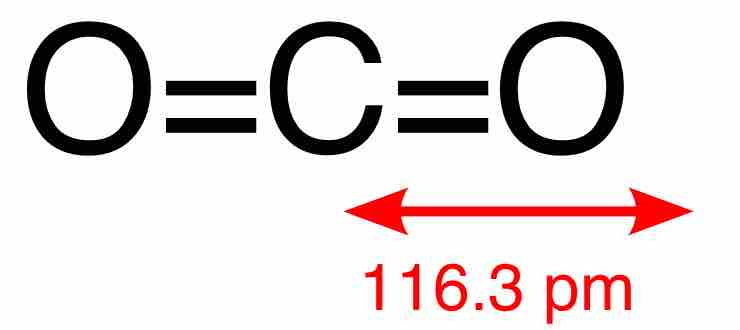
Carbon Dioxide
A central carbon atom is connected to two oxygen atoms via double bonds in a linear structure. The molecule has no net dipole moment because it's centrosymmetric.
Carbon dioxide in Earth's atmosphere currently occurs at an average concentration of about 390 parts per million by volume. Concentrations of the gas tend to fall during the northern spring and summer as plants consume the gas (during the process of photosynthesis), and rise during autumn and winter as plants go dormant, decay, or die. CO2 is an end product of the metabolism of organisms via the cellular respiration process, in which energy is obtained from the breaking down of sugars, fats, and amino acids. Despite the fact that the human body produces approximately 2.3 pounds of carbon dioxide per day, it is considered toxic, and concentrations up to 10 percent may cause suffocation.
Carbonic Acid and Its Conjugate Bases
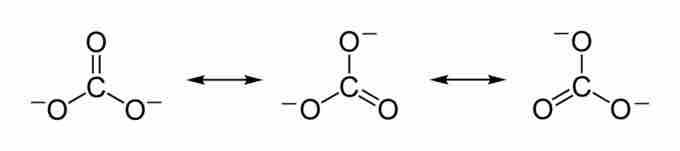
Carbon dioxide is soluble in water; it reversibly converts to carbonic acid (H2CO3). The salt of carbonic acids are called carbonates and are characterized by the carbonate ion, CO32-. The carbonate ion is the simplest oxocarbon anion, consisting of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement. The Lewis structure of the carbonate ion has two single bonds to negative oxygen atoms and one short double bond to a neutral oxygen. This structure is, however, incompatible with the ion's observed symmetry, which implies that the three bonds and oxygen atoms are equivalent. The symmetry can best be represented by three resonance structures.
The Carbonate Ion
The carbonate ion has three resonance structures. The true structure of the carbonate is an average of these three resonance structures.
In aqueous solutions, carbonate, bicarbonate (HCO3-), carbon dioxide, and carbonic acid exist together in equilibrium. In strongly basic conditions, the carbonate ion predominates, while in weakly basic conditions, the bicarbonate ion predominates. In acidic conditions, aqueous CO2 (aq) is the main form and is in equilibrium with carbonic acid -- the equilibrium lies strongly towards carbon dioxide.
Carbonate Salts
Metal carbonates generally decompose upon heating, liberating carbon dioxide and leaving behind an oxide of the metal. Ionic compounds form when a positively charged metal ion, M+, attaches to the negatively charged oxygen atoms of the carbonate ion. Most salts are insoluble in water, with solubility constants (Ksp) less than 1 x 10-8, with the exception of lithium, sodium, potassium, and ammonium carbonates.
Sodium carbonate is basic when dissolved in water (meaning it results in a basic solution upon dissolution), and sodium bicarbonate is weakly basic. These effects can be explained by considering that upon dissolution and subsequent dissociation of the salt into its ions, the carbonate or bicarbonate ions will react with H+ in the solution to form H2CO3 (which has a low Ka value - i.e., is a weak acid). On the other hand, carbon dioxide is weakly acidic (results in a slightly acidic solution) when dissolved in water. That's because it reacts with water to produce H2CO3, a small amount of which will dissociate into H+ and a bicarbonate ion.
Although the carbonate salts of most metals are insoluble in water, this is not true of the bicarbonate salts. Under changing temperature or pressure, and in the presence of metal ions with insoluble carbonates, the equilibrium between carbonate, bicarbonate, carbon dioxide, and carbonic acid in water can result in the formation of insoluble compounds. This is responsible for the buildup of scale inside pipes caused by hard water.