Phase Transition: Liquid to Gas
Vaporization of a sample of liquid is a phase transition from the liquid phase to the gas phase. There are two types of vaporization: evaporation and boiling.
- Evaporation occurs at temperatures below the boiling point, and occurs on the liquid's surface. For molecules of a liquid to evaporate, they must be located near the surface, be moving in the proper direction, and have sufficient kinetic energy to overcome intermolecular forces present in the liquid phase.
- Boiling, by contrast, is a rapid vaporization that occurs at or above the boiling temperature and at or below the liquid's surface.
Vapor Pressure
In much the same way that tea spreads out from a tea bag once the bag is immersed in water, molecules that are confined within a phase will tend to spread themselves (and the thermal energy they carry with them) as widely as possible. This fundamental law of nature is manifested in what is called the "escaping tendency" of the molecules from the phase. The escaping tendency is of fundamental importance in understanding all chemical equilibria and transformations.
It is possible to observe the tendency of molecules to escape into the gas phase from a solid or liquid by placing the substance in a closed, evacuated container connected to a manometer for measuring gas pressure.
If this is done with water, the partial pressure of water Pw in the space above the liquid will initially be zero (step 1). Gradually, Pw will rise as molecules escape from the liquid phase and enter the vapor phase. At the same time, some of the vapor molecules will condense back into the liquid phase (step 2). Because this latter process is less favorable (at the particular temperature represented here), Pw continues to rise as more water vapor forms. Eventually a balance is reached between the two processes (step 3), and Pw stabilizes at a fixed, or equilibrium, value Pvap , which depends on the substance and temperature. Pvap is known as the "equilibrium vapor pressure", or simply as the "vapor pressure" of the liquid. The vapor pressure is a direct measure of the escaping tendency of molecules from a condensed state of matter.
Boiling
The boiling point is the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding environment (air). The escaping tendency of molecules from a phase always increases with temperature; therefore, the vapor pressure of a liquid will be greater at higher temperatures. The variation of vapor pressure with temperature is not linear. Because the typical pressure at the earth's surface is 1 atm (760 torr), this is the vapor pressure that a liquid must equal in order for it to be at its normal boiling point.
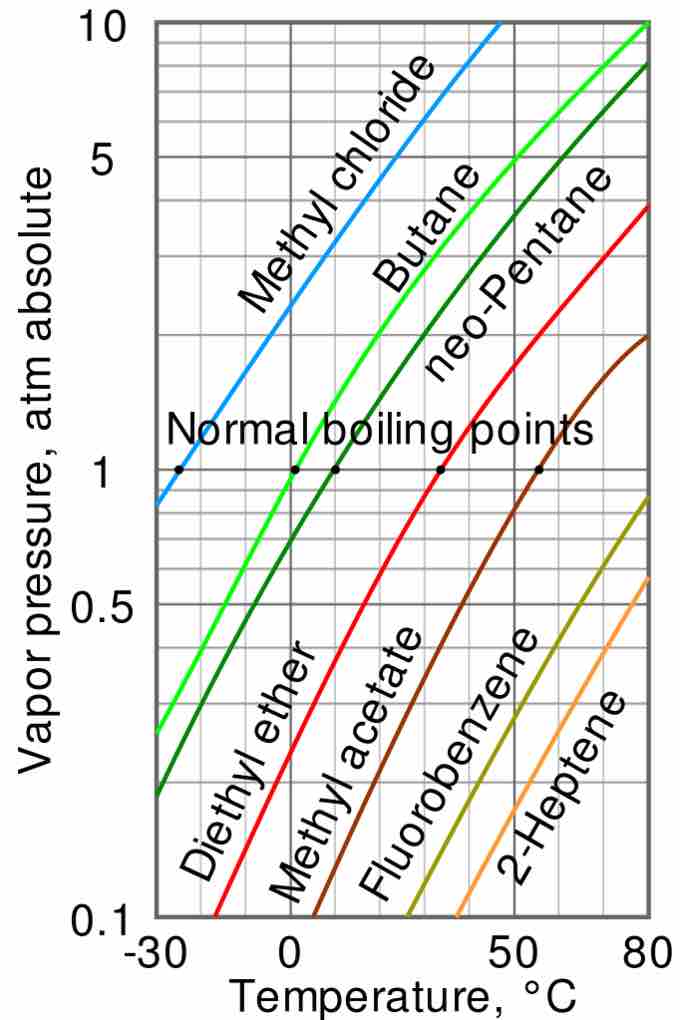
Vapor pressure and temperature
The variation of vapor pressure with temperature is not linear. The intercepts of each curve with the horizontal line at 1 atm (i.e. 760 torr) indicate the normal boiling point of each liquid, ranging from -25 °C for methyl chloride to over 80 °C for fluorobenzene and 2-heptene.
The curve suggests that when the atmospheric pressure is lower than 1 atm (for instance, at higher altitudes), then the boiling point will occur at lower temperatures. This is because the liquid can be heated less in order for its vapor pressure to equal the atmospheric pressure. This has indeed been observed to be true.
Related Phenomena Involving The Liquid Boundary Curvature
The vapor pressure of a liquid is determined by the attractive forces that act on the molecules at the surface of a liquid. In a very small drop, the liquid surface is curved in such a way that each molecule experiences fewer nearest-neighbor attractions than is the case for the bulk liquid. The outermost molecules of the liquid are bound to the droplet less tightly, and the drop has a larger vapor pressure than does the bulk liquid. If the vapor pressure of the drop is greater than the partial pressure of vapor in the gas phase, the drop will evaporate.
A bubble is a hole in a liquid; molecules at the liquid boundary are curved inward, so they experience stronger nearest-neighbor attractions. As a consequence, the vapor pressure Pw of the liquid facing into a bubble is always less than that of the bulk liquid Pw at the same temperature. When the bulk liquid is at its normal boiling point (that is, when its vapor pressure is 1 atm), the pressure of the vapor within the bubble will be less than 1 atm, so the bubble will tend to collapse. Also, since the bubble is formed within the liquid, the hydrostatic pressure of the overlaying liquid will add to this effect. For both of these reasons, a liquid will not boil until the temperature is raised slightly above the boiling point, a phenomenon known as superheating. Once the boiling begins, it will continue to do so at the liquid's proper boiling point.