Catalysts are chemical compounds that increase the rate of a reaction by lowering the activation energy required to reach the transition state. Unlike reactants, a catalyst is not consumed as part of the reaction process. The process of speeding up a reaction by using a catalyst is known as catalysis.
Types of Catalysts
Catalysts can be divided into two types, homogeneous or heterogeneous, depending on the reaction phase that they occupy. Homogeneous catalysts are those that occupy the same phase as the reaction mixture (typically liquid or gas), while heterogeneous catalysts occupy a different phase.
Generally, heterogeneous catalysts are solid compounds that are added to liquid or gas reaction mixtures. The reason such catalysts are able to speed up a reaction has to do with collision theory. Recall that according to collision theory, reactant molecules must collide with proper orientation. A catalyst essentially acts like a "traffic cop," aligning molecules in just the right way so that it's easier for them to combine and react.
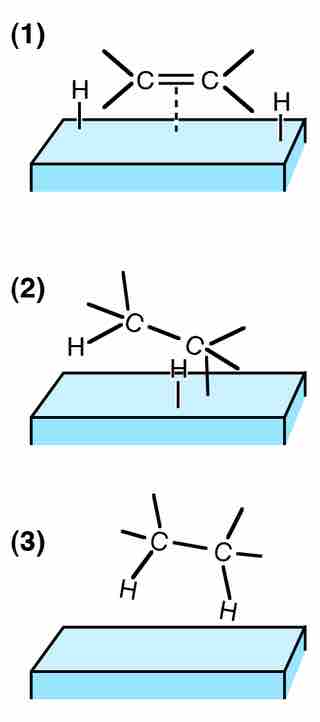
Adsorption of ethene on a solid catalyst surface
The catalyst aligns the ethene molecule with hydrogen, allowing the two to react more efficiently than if they were simply combined in a container, and were colliding at random.
Advantages and Disadvantages of Heterogeneous Catalysis
Heterogeneous catalysis has a number of benefits. For one, heterogeneous catalysts can be separated from a reaction mixture in a straightforward manner, such as by filtration. In this way, expensive catalysts can be easily and effectively recovered, which is an important consideration for industrial manufacturing processes.
However, one limitation of heterogeneous catalysis has to do with the available surface area of the catalyst. Once the surface of the catalyst is completely saturated with reactant molecules, the reaction cannot proceed until products leave the surface, and some space opens up again for a new reactant molecule to adsorb, or attach. It is for this reason that the adsorption step in a heterogeneously catalyzed reaction is oftentimes the rate-limiting step. Despite this, the overall benefits of heterogeneous catalysis often outweigh its disadvantages, in that the catalyzed reaction is still much faster than the uncatalyzed reaction.