The Octet Rule and Its Exceptions
The octet rule states that atoms below atomic number 20 tend to combine so that they each have eight electrons in their valence shells, which gives them the same electronic configuration as a noble gas. The rule is applicable to the main-group elements, especially carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium and magnesium.
Valence electrons can be counted using a Lewis electron dot diagram. In carbon dioxide, for example, each oxygen shares four electrons with the central carbon. These four electrons are counted in both the carbon octet and the oxygen octet because they are shared.

Carbon dioxide
A Lewis dot diagram for carbon dioxide.
Hydrogen and Lithium
However, many atoms below atomic number 20 often form compounds that do not follow the octet rule. For example, with the duet rule of the first principal energy level, the noble gas helium, He, has two electrons in its outer level. Since there is no 1p subshell, 1s is followed immediately by 2s, and thus level 1 can only have at most two valence electrons. Hydrogen only needs one additional electron to attain this stable configuration, through either covalent sharing of electrons or by becoming the hydride ion (:H-), while lithium needs to lose one by combining ionically with other elements. This leads to hydrogen and lithium both having two electrons in their valence shell—the same electronic configuration as helium—when they form molecules by bonding to other elements.
Boron and Aluminum
There are also a variety of molecules in which there are too few electrons to provide an octet for every atom. Boron and aluminum, from Group III (or 13), display different bonding behavior than previously discussed. These atoms each have three valence electrons, so we would predict that these atoms want to bond covalently in order to gain 5 electrons (through sharing) to fulfill the octet rule. However, compounds in which boron or aluminum atoms form five bonds are never observed, so we must conclude that simple predictions based on the octet rule are not reliable for Group III.
Consider boron trifluoride (BF3). The bonding is relatively simple to model with a Lewis structure if we allow each valence level electron in the boron atom to be shared in a covalent bond with each fluorine atom. In this compound, the boron atom only has six valence shell electrons, but the octet rule is satisfied by the fluorine atoms.
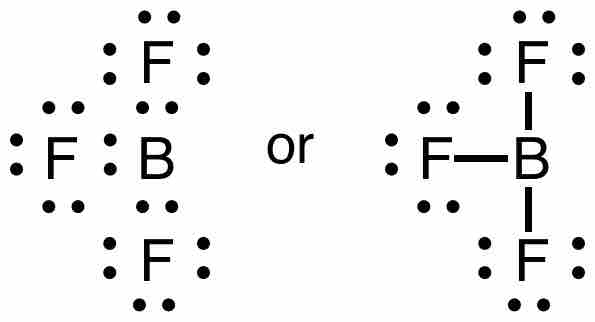
Lewis structure of boron trifluoride
Each pair of dots represents a pair of electrons. When placed between two atoms, the electrons are in a bond. A bond can be drawn as a line between two atoms, which also indicates two electrons. Notice that the central boron atom has only 6 electrons in the final Lewis diagram/structure of this molecule.
We might conclude from this one example that boron atoms obey a sextet rule. However, boron will form a stable ion with hydrogen, BH4-, in which the boron atom does have a complete octet. In addition, BF3 will react with ammonia (NH3), to form a stable compound, NH3BF3, for which a Lewis structure can be drawn that shows boron with a complete octet.
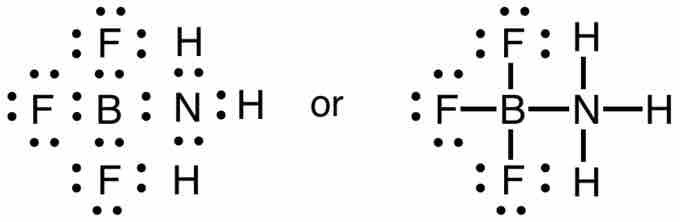
Boron trifluoride-ammonia complex
This covalent compound (NH3BF3) shows that boron can have an octet of electrons in its valence level.
Compounds of aluminum follow similar trends. Aluminum trichloride (AlCl3), aluminum hydride (AlH3), and aluminum hydroxide (Al(OH)3) indicate a valence of three for aluminum, with six valence electrons in the bonded molecule. However, the stability of aluminum hydride ions (AlH4-) indicates that Al can also support an octet of valence shell electrons.
Although the octet rule can still be of some utility in understanding the chemistry of boron and aluminum, the compounds of these elements are harder to predict than for other elements.