Lewis dot structures can be drawn to visualize the electrons and bonds of a certain molecule. However, for some molecules not all the bonding possibilities cannot be represented by a single Lewis structure; these molecules have several contributing or "resonance" structures. In chemistry terms, resonance describes the fact that electrons are delocalized, or flow freely through the molecule, which allows multiple structures to be possible for a given molecule.
Each contributing resonance structure can be visualized by drawing a Lewis structure; however, it is important to note that each of these structures cannot actually be observed in nature. That is, the molecule does not actually go back and forth between these configurations; rather, the true structure is an approximate intermediate between each of the structures. This intermediate has an overall lower energy than each of the possible configurations and is referred to as a resonance hybrid. It is important to note that the difference between each structure lies in the location of the electrons and not in the arrangement of the atoms.
More Than One Valid Lewis Structure
For example, the nitrate ion, NO3-, has more than one valid Lewis structure. The structure contains two N-O single bonds and one N=O double bond. But the question then remains as to which oxygen should be involved in the double bond. Therefore, three valid resonance structures can be drawn. Double-ended arrows are used to indicate that the structures are chemically equivalent. Again, in reality, the electronic configuration does not change between the three structures; rather, it has one structure in which the extra electrons are distributed evenly. These fractional bonds are sometimes depicted by dashed arrows, which show that the electron density is spread out throughout the compound.
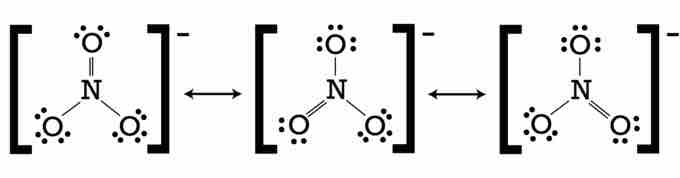
Resonance structures of the nitrate ion
The nitrate ion has three valid contributing structures that vary according to the placement of the electrons.
Drawing Resonance Structures
When you are drawing resonance structures, it is important to remember to shift only the electrons; the atoms must have the same position. Sometimes, resonance structures involve the placement of positive and negative charges on specific atoms. Because atoms with electric charges are not as stable as atoms without electric charges, these resonance structures will contribute less to the overall resonance structure than a structure with no charges.