Free Radicals
Some elements, most notably nitrogen, can form compounds that do not obey the octet rule. One class of such compounds are those that have an odd number of electrons. As the octet rule requires eight electrons around each atom, a molecule with an odd number of electrons must disobey the octet rule. Molecules with unpaired electrons are termed 'free radicals.' While typically highly unstable, and therefore highly reactive, some free radicals exhibit stability of days, months, or even years. These latter compounds are said to be 'metastable,' meaning they will decompose or react if given enough time, but are stable enough for a considerable amount of time, from days to even years, when subjected to only minor disturbances.
Examples of Free Radical Molecules
Recall that the Lewis structure of a molecule must depict the total number of valence electrons from all the atoms which are bonded together.
Nitric oxide has the formula NO. The total number of valence electrons is 5+6=11. Therefore, no matter how electrons are shared between the nitrogen and oxygen atoms, there is no way for nitrogen to have an octet. It will have seven electrons, assuming that the oxygen atom does satisfy the octet.
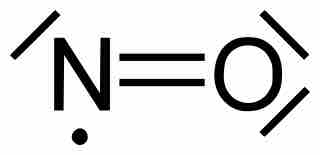
Nitric oxide
Nitric oxide (NO) is an example of a stable free radical. It does not obey the octet rule on the nitrogen atom. Each line around the atoms represents a pair of electrons.
Nitric oxide is a by-product of combustion reactions that occur in engines, like those in automobile engines and fossil fuel power plants. It is also produced naturally during the electrical discharge of lightning during thunderstorms.
Nitrogen dioxide is the chemical compound with the formula NO2. Again, nitrogen dioxide does not follow the octet rule for one of its atoms, namely nitrogen. The total number of valence electrons is 5+2(6)=17. There is persistent radical character on nitrogen because it has an unpaired electron. The two oxygen atoms in this molecule follow the octet rule.

Nitrogen dioxide
Nitrogen dioxide is another stable molecule that disobeys the octet rule. Note the seven electrons around nitrogen. Formal charges and the molecule's resonance structures are indicated.
Nitrogen dioxide is an intermediate in the industrial synthesis of nitric acid, millions of tons of which is produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent air pollutant.