Concept
Version 14
Created by Boundless
Balancing Redox Equations
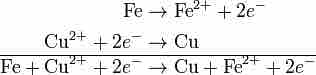
Adding the two halves of a redox reaction
These two halves of the reaction can be added like any other chemical equation. Once the equations are added, the electrons on each side cancel out.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"General Chemistry/Redox Reactions/Oxidation and Reduction equations."
http://en.wikibooks.org/wiki/General_Chemistry/Redox_Reactions/Oxidation_and_Reduction_equations
Wikibooks
CC BY-SA 3.0.