Salts With a Hydrolyzable Cation
When dissolved in water, acidic salts will yield solutions with pH less than 7.0. This is due either to the presence of a metal cation that acts as a Lewis acid (which will be discussed in a later concept), or, quite commonly, due to a hydrolyzable proton in the cation or the anion. Salts with acidic protons in the cation are most commonly ammonium salts, or organic compounds that contain a protonated amine group. Examples include:
- ammonium (NH4+)
- methyl ammonium (CH3NH3+)
- ethyl ammonium (CH3CH2NH3+)
- anilinium (C6H6NH2+)
An example of an acid salt is one containing any of these cations with a neutral base, such as ammonium chloride (NH4Cl).
Salts With Hydrolyzable Protons in the Anion
Acid salts can also contain an acidic proton in the anion. Examples of anions with an acidic proton include:
- bisulfate (HSO4-)
- dihydrogen citrate (H2C6H5O7-)
- bioxalate (HO2C2O-)
Each of these anions contains a proton that will weakly dissociate in water. Therefore, salts containing these anions—such as potassium bisulfate—will yield weakly acidic solutions in water.
Determining Acidity or Alkalinity of a Hydrolyzable Ion
From the previous concept, we know that salts containing the bicarbonate ion (HCO3-) are basic, whereas salts containing bisulfate ion (HSO4-) are acidic. We determine whether the hydrolyzable ion is acidic or basic by comparing the Ka and Kb values for the ion; if Ka > Kb, the ion will be acidic, whereas if Kb > Ka, the ion will be basic.
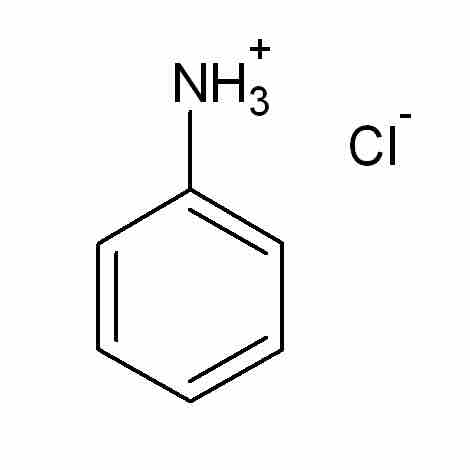
Anilinium chloride
Anilinium chloride is an example of an acid salt. The NH3+ group contains an acidic proton capable of dissociating in solution; therefore, a solution of anilinium chloride in pure water will have a pH less than 7.