Total synthesis is a field of research in organic chemistry that focuses on the synthesis of large, typically biologically useful compounds from simple, common materials. It is the academic foundation of the pharmaceutical industry.
Linear vs piecewise synthesis
Laboratory synthesis of a large molecule can be very difficult, costly and time-consuming. The reactions themselves can take over 24 hours, and isolation, purification and characterization can also take several more hours. In order to minimize risk of losing material in a failed reaction and to use time efficiently, a piecewise synthesis scheme is often used.
In linear synthesis, a chemist begins with a single starting material A, and performs many sequential reactions to produce target material B. If a 95% yield can be achieved with each step, an eight-step synthesis affords a net yield of 66% (0.958*100%). Because each step occurs in sequence, it is impossible to do two reactions simultaneously towards the same linear synthesis. It also should be noted that if a reaction fails, it's possible that no useful material may be recovered and the sequence will have to be started from step 1.
Piecewise synthesis involves breaking target material B into several pieces that can be synthesized separately, and then combining them. If B can be synthesized from W, X, Y, and Z, and each of W, X, Y, and Z can be synthesized in one step, it saves both time and materials. Assuming 95% yield for each step, each of W, X, Y and Z are produced from starting materials in 95% yield. Combining them sequentially requires three steps (W+X, then add Y, then Z), which results in an 86% yield (0.953*100%). A savvy chemist can have syntheses for W, X, Y, and Z simultaneously running, thus decreasing time to complete the total synthesis.
Chemoselectivity and protecting groups
One of the greatest challenges in total synthesis is selective reactivity. Oftentimes, chemists will use chemoselective reactions that are highly discriminatory between slight differences in functional groups with which they react.
For example, a molecule containing an imine, carboxylic acid and ketone will have all three of those functional groups reduced by lithium aluminum hydride. Sodium borohydride will reduce only the imine and ketone. But what if a chemist's intention is to leave the carboxylic acid and ketone functionalities, while reducing the imine? Sodium cyanoborohydride is chemoselective enough to reduce only the imine.
Another tactic for chemists is to use protecting groups to temporarily block a reactive site. Consider the reaction:
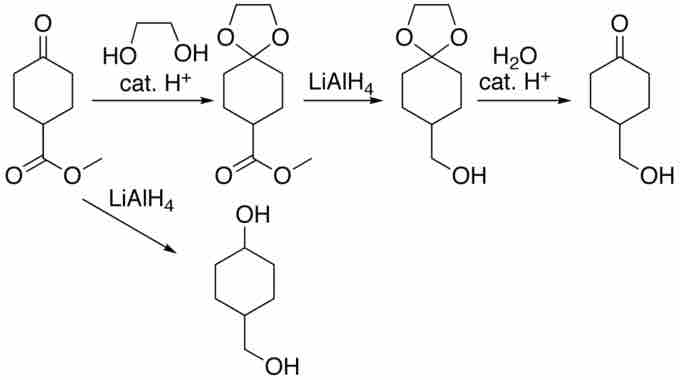
Acetal Protection of a Ketone
There is no reducing agent that would reduce the ester to an alcohol that would not also reduce the ketone. However, the ketone can form an acetal which is unaffected by lithium aluminum hydride. Once the ester is reduced, the acetal can be removed, thus yielding the original ketone functionality.
Protecting groups can be extremely useful, but to be worthwhile, must use inexpensive materials and must be easily added and removed in high yield.
Stereoselectivity
Perhaps the most difficult and important challenge modern chemists face is stereoselective synthesis. Especially in pharmaceuticals, it's necessary to produce compounds that are enantiomerically pure. One enantiomer may have medicinal properties, while the other may be entirely useless or worse, have adverse effects.
Thalidomide , a drug used to relieve morning sickness in pregnant women in the 1950s, was initially sold as a racemic mixture of enantiomers. The R-enantiomer has no adverse effects, but the S-enantiomer is a teratogen that caused horrible birth defects during clinical testing.
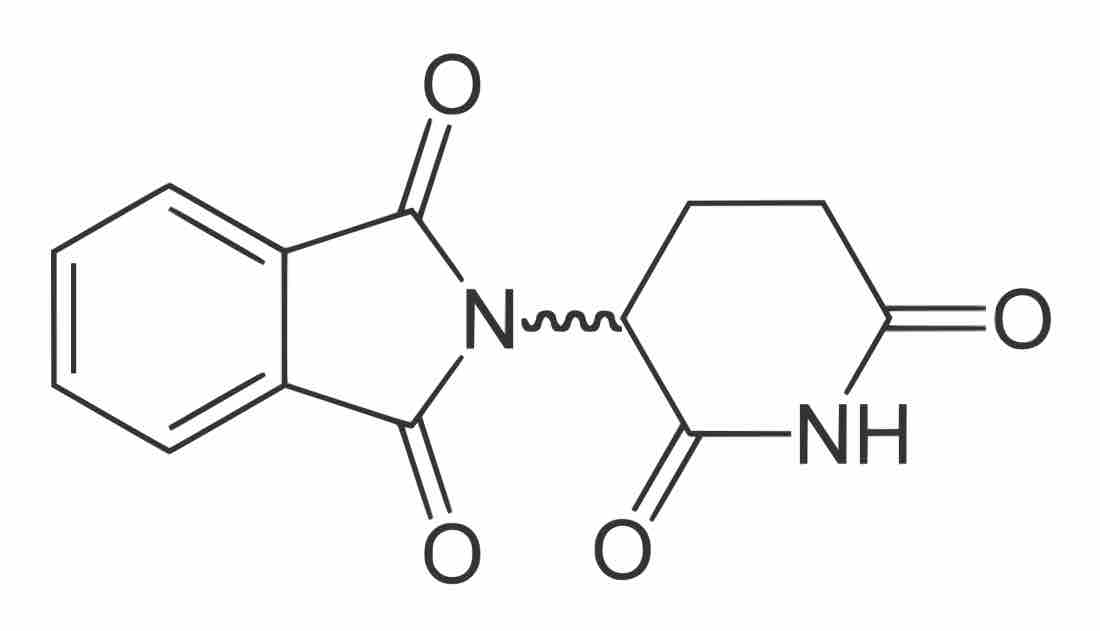
Thalidomide
Stereoselectivity in organic synthesis is a broad topic that is worthy of its own graduate-level college course, so for the purposes of this atom, we will skim the surface of the topic, which can be broken into enantioselectivity and diastereoselectivity.
An enantioselective reaction is one in which one enantiomer is preferred over another in a reaction. Because both enantiomers of any compound have identical physical and chemical properties, there must be another influence to favor one product over the other. This is usually a chiral auxiliary, which can block one face of a stereocenter, forcing the reaction to occur only from the other side. The addition of a chiral auxiliary that can easily be removed exploits the chemically different properties from one diastereomer to another, which is the foundation of diastereoselectivity.
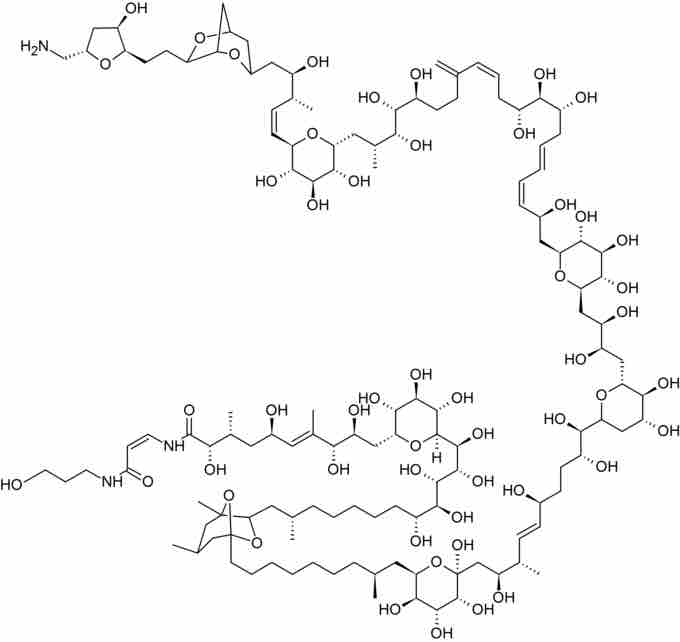
Palytoxin
The enantioselective synthesis of palytoxin, a natural product with 64 stereocenters, is widely regarded as the greatest-ever achievement in total synthesis.