Alcohols are ubiquitous in nature and can be formed and augmented in many different ways. This atom discusses their synthesis and reactions.
Alcohol Synthesis
Nucleophilic Substitution
Nucleophilic substitution is a common example, with SN2 conditions working best for primary alcohol synthesis and SN1 conditions affording secondary and especially tertiary alcohols.
A common primary alcohol synthesis via SN2 is:
R-CH2Br + OH- → R-CH2OH + Br-
A common tertiary alcohol synthesis via SN1 is:
R3CBr + H2O → R3COH + Br-
Reduction
Reduction of ketones and aldehydes affords secondary and primary alcohols, respectively. Hydride-containing reducing agents like NaBH4 and LiAlH4 (with acid workup) produce alcohols. For example, consider the reduction of the aldehyde:
RCHO + NaBH4 → RCH2OH
Under stronger conditions, carboxylic acids, esters, acid anhydrides, and acyl halides can also be reduced to primary acids. LiAlH4 can perform the reduction.
Hydration
Alkenes can be hydrated in acidic conditions, in which case the elements of water are added across a double bond that is broken. The mechanism includes three steps: 1) protonation; 2) nucleophilic addition of water to the resulting carbocation to form a protonated alcohol; 3) deprotonation of the protonated alcohol. Stepwise, the general reaction is:
R-CH=CH2 + H2SO4 + H2O RCH+-CH3 + H2O RCHOH2+CH3
Deprotonation from the solvent yields: RCHOHCH3
The product of acid-catalyzed hydration is called a "Markownikoff" product: because the carbocation must be on the most substituted carbon (where it is most stable), this is where the hydroxyl adds. To produce an anti-Markownikoff product, in which the alcohol is formed on the less substituted end, hydroboration, followed by oxidation, can be used.
Reacting an alkene with BH3 will add a hydride onto the more substituted carbon, and BH2 onto the less substituted carbon. The resulting organoborane can be oxidized by H2O2 in basic conditions to remove the -BH2, and replace it with -OH:
R-CH=CH2 + BH3 R-CH2-CH2BH2
R-CH2-CH2BH2 +H2O2 + OH- R-CH2-CH2OH
Reactions of Alcohols
Alkoxide formation
Alcohols can be deprotonated in the presence of bases to form alkoxide salts:
ROH + NaH RO- Na+ + H2
Williamson ether synthesis
Alkoxides can be used to form ethers by reacting with leaving group-containing alkanes. The Williamson ether synthesis involves an alkoxide nucleophile reacting with an electrophilic alkyl halide to produce an ether. For example:
RO- Na+ + R'Cl ROR' + Na+Cl-
Elimination
Alcohols can undergo elimination, but only by E1. In basic conditions, which are required for E2, alcohols are deprotonated (see alkoxide formation) before they can be eliminated.
The classic example of alcohol elimination is an exact reversal of hydration. In the presence of acid, the alcohol is first protonated, then dissociates, leaving a carbocation. A weak base (typically a solvent) then deprotonates the most-substituted vicinal carbon, affording an e-isomer product. The reaction can be modeled as:
RCHOHCH3 + H2SO4 RCHOH2+CH3 RCH+CH3
From here, the water produced can deprotonate the carbocation to form a double bond:
RCH+CH3 + H2O R-CH=CH2 + H3O+
Alkyl halide formation
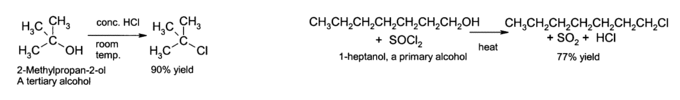
SN1 and SN2 Synthesis of Alkyl Halides From Alcohols
On the left, a tertiary alcohol undergoes an SN1 reaction. On the right, a primary alcohol undergoes an SN2 reaction. Both afford alkyl halides.
Alcohols can be converted to alkyl halides by SN1 and SN2 modes of nucleophilic substitution. Both modes can occur using similar conditions. In the case of halogenating a primary alcohol, SN2 can be achieved by using a hydrohalic acid. The acid protonates the alcohol to make it a good leaving group, then the halide displaces the alcohol with nucleophilic attack on the electrophilic carbon. An example of such a reaction is:
ROH + HCl ROH2+ + Cl- RCl + H2O
A similar reaction can be performed using PBr3 or SOCl2 (for bromination and chlorination, respectively) as agents to make the hydroxyl more labile.
Secondary and tertiary alcohols can undergo SN1 nucleophilic substitution when exposed to hydrohalic acids. The acid first protonates the alcohol before the reaction continues with a classic SN1 mechanism.
Esterification
Alcohols can react with carboxylic acids, acid anhydrides, and acyl halides to form esters. The classic esterification is the Fischer esterification, which is the acid-catalyzed reaction of a carboxylic acid and alcohol and has the general form:
ROH + R'CO2H + H+ (cat.) RCO2C' + H2O
Oxidation
Alcohols can be oxidized to ketones, aldehydes, and carboxylic acids depending on their substitution and reaction conditions. Although tertiary alcohols cannot be oxidized, secondary alcohols can be converted to ketones using an oxidizing agent. KMnO4 and K2Cr2O7 can, in acid, oxidize secondary alcohols to ketones, as can CrO3 in the presence of peracetic acid.
Primary alcohols can be oxidized to aldehydes or carboxylic acids. MnO2, pyridinium chlorochromate and the Sarett-Collins reagent will afford the aldehyde, while acidic solutions of KMnO4 or K2Cr2O7 beget the carboxylic acid.