Consider two reactions: the rusting of an iron nail and the combustion of propane. Both reactions can occur, and both will occur to completion. The rusting will take years to complete, but propane will combust in an instant. Furthermore, the nail will rust faster when it is moist, and it will rust more slowly in the presence of less oxygen.
Obviously, there are factors that affect the rates of chemical reactions. Knowing how quickly a chemical reaction occurs is a crucial factor in how the reaction affects its surroundings and vice versa. The study of these factors and rates is known as chemical kinetics.
The interval required for a chemical change or reaction to occur is called the reaction time. Every reaction has its own unique reaction time. Each reaction also has a reaction rate. Reaction rates are not usually constant over a given reaction time. As described in previous sections, the reaction reaches equilibrium when its rate is constant, as seen in .
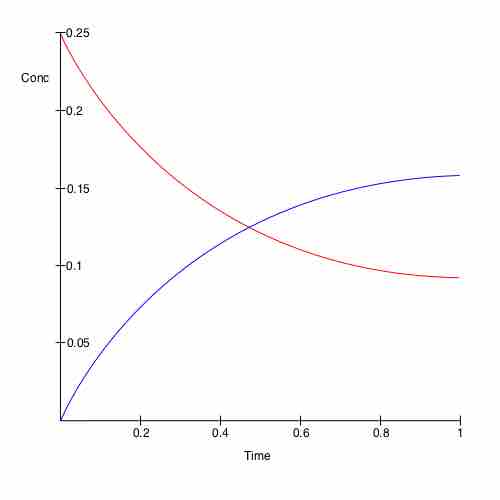
Change in concentration of chemicals over time
A plot of time versus concentration for two species in chemical equilibrium.
Consider this generic chemical reaction:
The rate at which products are formed and reactants are used up determines the speed of the reaction. This rate can be expressed as the ratio of the change in the concentration of a reactant (or product) to the change in time.
The reaction rate involves differential equations, but in non-mathematical terms it is simply the rate of change in the concentrations. Since it is customary to define rates as positive quantities and the change in the concentration of the reactants is negative, the values of [A] and [B] are preceded by minus signs so that the final result will be positive. The unit of this rate is usually M/second.
Actually measuring the rates of change in the reactants and products is difficult. Instead, the reaction rate can be accurately modeled by a rate equation. This is an example of a rate equation that might model the above reaction:
where k is a constant. The reaction rate can be determined using a rate law, which depends on the concentrations of the reactants, among other things. You can read more about reaction rates and rate laws in the Kinetics unit.