The End Problem of Linear DNA Replication
Linear chromosomes have an end problem. After DNA replication, each newly synthesized DNA strand is shorter at its 5' end than at the parental DNA strand's 5' end. This produces a 3' overhang at one end (and one end only) of each daughter DNA strand, such that the two daughter DNAs have their 3' overhangs at opposite ends
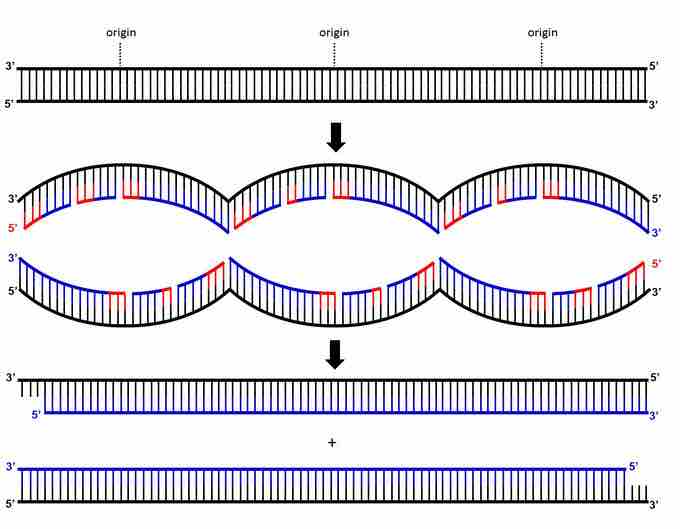
The telomere end problem
A simplified schematic of DNA replication where the parental DNA (top) is replicated from three origins of replication, yielding three replication bubbles (middle) before giving rise to two daughter DNAs (bottom). Parental DNA strands are black, newly synthesized DNA strands are blue, and RNA primers are red. All RNA primers will be removed by Rnase H and FEN1, leaving gaps in the newly-synthesized DNA strands (not shown.) DNA Polymerase and Ligase will replace all the RNA primers with DNA except the RNA primer at the 5' ends of each newly-synthesized (blue) strand. This means that each newly-synthesized DNA strand is shorter at its 5' end than the equivalent strand in the parental DNA.
Every RNA primer synthesized during replication can be removed and replaced with DNA strands except the RNA primer at the 5' end of the newly synthesized strand. This small section of RNA can only be removed, not replaced with DNA. Enzymes RNase H and FEN1 remove RNA primers, but DNA Polymerase will add new DNA only if the DNA Polymerase has an existing strand 5' to it ("behind" it) to extend. However, there is no more DNA in the 5' direction after the final RNA primer, so DNA polymerse cannot replace the RNA with DNA. Therefore, both daughter DNA strands have an incomplete 5' strand with 3' overhang.
In the absence of additional cellular processes, nucleases would digest these single-stranded 3' overhangs. Each daughter DNA would become shorter than the parental DNA, and eventually entire DNA would be lost. To prevent this shortening, the ends of linear eukaryotic chromosomes have special structures called telomeres.
Telomere Replication
The ends of the linear chromosomes are known as telomeres: repetitive sequences that code for no particular gene. These telomeres protect the important genes from being deleted as cells divide and as DNA strands shorten during replication.
In humans, a six base pair sequence, TTAGGG, is repeated 100 to 1000 times. After each round of DNA replication, some telomeric sequences are lost at the 5' end of the newly synthesized strand on each daughter DNA, but because these are noncoding sequences, their loss does not adversely affect the cell. However, even these sequences are not unlimited. After sufficient rounds of replication, all the telomeric repeats are lost, and the DNA risks losing coding sequences with subsequent rounds.
The discovery of the enzyme telomerase helped in the understanding of how chromosome ends are maintained. The telomerase enzyme attaches to the end of a chromosome and contains a catalytic part and a built-in RNA template. Telomerase adds complementary RNA bases to the 3' end of the DNA strand. Once the 3' end of the lagging strand template is sufficiently elongated, DNA polymerase adds the complementary nucleotides to the ends of the chromosomes; thus, the ends of the chromosomes are replicated.
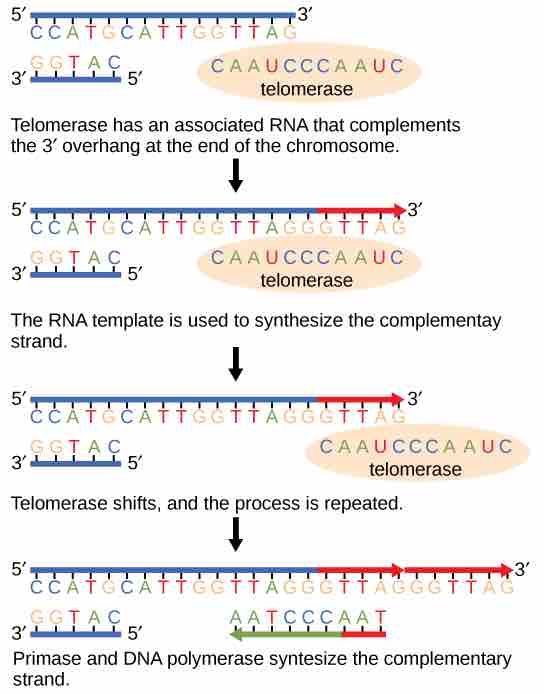
Telomerase is important for maintaining chromosome integrity
The ends of linear chromosomes are maintained by the action of the telomerase enzyme.
Telomerase and Aging
Telomerase is typically active in germ cells and adult stem cells, but is not active in adult somatic cells. As a result, telomerase does not protect the DNA of adult somatic cells and their telomeres continually shorten as they undergo rounds of cell division.
In 2010, scientists found that telomerase can reverse some age-related conditions in mice. These findings may contribute to the future of regenerative medicine. In the studies, the scientists used telomerase-deficient mice with tissue atrophy, stem cell depletion, organ failure, and impaired tissue injury responses. Telomerase reactivation in these mice caused extension of telomeres, reduced DNA damage, reversed neurodegeneration, and improved the function of the testes, spleen, and intestines. Thus, telomere reactivation may have potential for treating age-related diseases in humans.