Sodium sulfate
About this schools Wikipedia selection
This content from Wikipedia has been selected by SOS Children for suitability in schools around the world. SOS mothers each look after a a family of sponsored children.
| Sodium sulfate | |
|---|---|
 |
 |
|
Other names
Thenardite (mineral) |
|
| Identifiers | |
| CAS number | 7757-82-6 7727-73-3 (decahydrate) |
| PubChem | 24436 |
| ChemSpider | 22844 |
| UNII | 36KCS0R750 |
| ChEBI | CHEBI:32149 |
| ChEMBL | CHEMBL233406 |
| RTECS number | WE1650000 |
| ATC code | A06, A12 CA02 |
| Jmol-3D images | Image 1 |
|
SMILES
|
|
|
InChI
|
|
| Properties | |
| Molecular formula | Na2SO4 |
| Molar mass | 142.04 g/mol (anhydrous) 322.20 g/mol (decahydrate) |
| Appearance | white crystalline solid hygroscopic |
| Odour | odorless |
| Density | 2.664 g/cm3 (anhydrous) 1.464 g/cm3 (decahydrate) |
| Melting point |
884 °C (anhydrous) |
| Boiling point |
1429 °C (anhydrous) |
| Solubility in water | anhydrous: 4.76 g/100 mL (0 °C) 42.7 g/100 mL (100 °C) heptahydrate: 19.5 g/100 mL (0 °C) 44 g/100 mL (20 °C) |
| Solubility | insoluble in ethanol soluble in glycerol and hydrogen iodide |
| Refractive index (nD) | 1.468 (anhydrous) 1.394 (decahydrate) |
| Structure | |
| Crystal structure | orthorhombic or hexagonal (anhydrous) monoclinic (decahydrate) |
| Hazards | |
| MSDS | External MSDS |
| EU Index | Not listed |
| Main hazards | Irritant |
| NFPA 704 | |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Sodium selenate Sodium tellurate |
| Other cations | Lithium sulfate Potassium sulfate Rubidium sulfate Caesium sulfate |
| Related compounds | Sodium bisulfate Sodium sulfite Sodium persulfate |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Sodium sulfate is the sodium salt of sulfuric acid. When anhydrous, it is a white crystalline solid of formula Na2SO4 known as the mineral thenardite; the decahydrate Na2SO4·10H2O has been known as Glauber's salt or, historically, sal mirabilis since the 17th century. Another solid is the heptahydrate, which transforms to mirabilite when cooled. With an annual production of 6 million tonnes, it is a major commodity chemical product.
Sodium sulfate is mainly used for the manufacture of detergents and in the Kraft process of paper pulping. About two-thirds of the world's production is from mirabilite, the natural mineral form of the decahydrate, and the remainder from by-products of chemical processes such as hydrochloric acid production.
History
The hydrate of sodium sulfate is known as Glauber's Salt after the Dutch/German chemist and apothecary Johann Rudolf Glauber (1604–1670), who discovered it in 1625 in Austrian spring water. He named it sal mirabilis (miraculous salt), because of its medicinal properties: the crystals were used as a general purpose laxative, until more sophisticated alternatives came about in the 1900s.
In the 18th century, Glauber's salt began to be used as a raw material for the industrial production of soda ash ( sodium carbonate), by reaction with potash ( potassium carbonate). Demand for soda ash increased and supply of sodium sulfate had to increase in line. Therefore, in the nineteenth century, the large scale Leblanc process, producing synthetic sodium sulfate as a key intermediate, became the principal method of soda ash production.
Physical and chemical properties
Sodium sulfate is chemically very stable, being unreactive toward most oxidising or reducing agents at normal temperatures. At high temperatures, it can be converted to sodium sulfide by carbothermal reduction:
- Na2SO4 + 2 C → Na2S + 2 CO2
Acid-base
Sodium sulfate is a neutral salt, which forms aqueous solutions with pH of 7. The neutrality of such solutions reflects the fact that sulfate is derived, formally, from the strong acid sulfuric acid. Furthermore, the Na+ ion, with only a single positive charge, only weakly polarizes its water ligands. Sodium sulfate reacts with sulfuric acid to give the acid salt sodium bisulfate:
- Na2SO4 + H2SO4 ⇌ 2 NaHSO4
The equilibrium constant for this process depends on concentration and temperature.
Solution and ion exchange
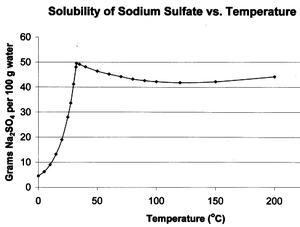
Sodium sulfate has unusual solubility characteristics in water. Its solubility in water rises more than tenfold between 0 °C to 32.384 °C, where it reaches a maximum of 497 g/L. At this point the solubility curve changes slope, and the solubility becomes almost independent of temperature. This temperature at 32.384 °C, corresponding to the release of crystal water and melting of the hydrated salt, serves as an accurate temperature reference for thermometer calibration.
Sodium sulfate is a typical ionic sulfate, containing Na+ ions and SO42− ions. The existence of sulfate in solution is indicated by the easy formation of insoluble sulfates when these solutions are treated with Ba2+ or Pb2+ salts:
- Na2SO4 + BaCl2 → 2 NaCl + BaSO4
Sodium sulfate displays a moderate tendency to form double salts. The only alums formed with common trivalent metals are NaAl(SO4)2 (unstable above 39 °C) and NaCr(SO4)2, in contrast to potassium sulfate and ammonium sulfate which form many stable alums. Double salts with some other alkali metal sulfates are known, including Na2SO4·3K2SO4 which occurs naturally as the mineral glaserite. Formation of glaserite by reaction of sodium sulfate with potassium chloride has been used as the basis of a method for producing potassium sulfate, a fertiliser. Other double salts include 3Na2SO4·CaSO4, 3Na2SO4·MgSO4 ( vanthoffite) and NaF·Na2SO4.
Structure
Crystals consist of [Na(OH2)6]+ ions with octahedral molecular geometry, as seen for many metallic sulfate salts. These cations are linked to the sulfate anions via hydrogen bonds. The Na-O distances are 240 pm. Two molecules of water per formula unit are not coordinated to Na+. Crystalline sodium sulfate decahydrate is also unusual among hydrated salts in having a measureable residual entropy (entropy at absolute zero) of 6.32 J·K−1·mol−1. This is ascribed to its ability to distribute water much more rapidly compared to most hydrates.
Production
The world production of sodium sulfate, mostly in the form of the decahydrate amounts to approximately 5.5 to 6 million tonnes annually (Mt/a). In 1985, production was 4.5 Mt/a, half from natural sources, and half from chemical production. After 2000, at a stable level until 2006, natural production had increased to 4 Mt/a, and chemical production decreased to 1.5 to 2 Mt/a, with a total of 5.5 to 6 Mt/a. For all applications, naturally produced and chemically produced sodium sulfate are practically interchangeable.
Chemical industry
About one third of the world's sodium sulfate is produced as by-product of other processes in chemical industry. Most of this production is chemically inherent to the primary process, and only marginally economical. By effort of the industry, therefore, sodium sulfate production as by-product is declining.
The most important chemical sodium sulfate production is during hydrochloric acid production, either from sodium chloride (salt) and sulfuric acid, in the Mannheim process, or from sulfur dioxide in the Hargreaves process. The resulting sodium sulfate from these processes are known as salt cake.
- Mannheim: 2 NaCl + H2SO4 → 2 HCl + Na2SO4
- Hargreaves: 4 NaCl + 2 SO2 + O2 + 2 H2O → 4 HCl + 2 Na2SO4
The second major production of sodium sulfate are the processes where surplus sulfuric acid is neutralised by sodium hydroxide, as applied on a large scale in the production of rayon. This method is also a regularly applied and convenient laboratory preparation.
- 2 NaOH( aq) + H2SO4(aq) → Na2SO4(aq) + 2 H2O(l)
In the laboratory it can also be synthesized from the reaction between sodium bicarbonate and magnesium sulfate.
- 2NaHCO3 + MgSO4 → Na2SO4 + Mg(OH)2 + 2CO2
Formerly, sodium sulfate was also a by-product of the manufacture of sodium dichromate, where sulfuric acid is added to sodium chromate solution forming sodium dichromate, or subsequently chromic acid. Alternatively, sodium sulfate is or was formed in the production of lithium carbonate, chelating agents, resorcinol, ascorbic acid, silica pigments, nitric acid, and phenol.
Bulk sodium sulfate is usually purified via the decahydrate form, since the anhydrous form tends to attract iron compounds and organic compounds. The anhydrous form is easily produced from the hydrated form by gentle warming.
Major sodium sulfate by-product producers of 50–80 Mt/a in 2006 include Elementis Chromium (chromium industry, Castle Hayne, NC, US), Lenzing AG (200 Mt/a, rayon industry, Lenzing, Austria), Addiseo (formerly Rhodia, methionine industry, Les Roches-Roussillon, France), Elementis (chromium industry, Stockton-on-Tees, UK), Shikoku Chemicals (Tokushima, Japan) and Visko-R (rayon industry, Russia).
Applications
Commodity industries
With US pricing at $30 per tonne in 1970, in 2006 up to $90 per tonne for salt cake quality and $130 for better grades, sodium sulfate is a very cheap material. The largest use is as filler in powdered home laundry detergents, consuming approx. 50% of world production. This use is waning as domestic consumers are increasingly switching to compact or liquid detergents that do not include sodium sulfate.
Another formerly major use for sodium sulfate, notably in the US and Canada, is in the Kraft process for the manufacture of wood pulp. Organics present in the "black liquor" from this process are burnt to produce heat, needed to drive the reduction of sodium sulfate to sodium sulfide. However, this process is being replaced by newer processes; use of sodium sulfate in the US and Canadian pulp industry declined from 1.4 Mt/a in 1970 to only approx. 150,000 tonnes in 2006.
The glass industry provides another significant application for sodium sulfate, as second largest application in Europe. Sodium sulfate is used as a fining agent, to help remove small air bubbles from molten glass. It fluxes the glass, and prevents scum formation of the glass melt during refining. The glass industry in Europe has been consuming from 1970 to 2006 a stable 110,000 tonnes annually.
Sodium sulfate is important in the manufacture of textiles, particularly in Japan, where it is the largest application. Sodium sulfate helps in "levelling", reducing negative charges on fibres so that dyes can penetrate evenly. Unlike the alternative sodium chloride, it does not corrode the stainless steel vessels used in dyeing. This application in Japan and US consumed in 2006 approximately 100,000 tonnes.
Thermal storage
The high heat storage capacity in the phase change from solid to liquid, and the advantageous phase change temperature of 32 °C (90 °F) makes this material especially appropriate for storing low grade solar heat for later release in space heating applications. In some applications the material is incorporated into thermal tiles that are placed in an attic space while in other applications the salt is incorporated into cells surrounded by solar–heated water. The phase change allows a substantial reduction in the mass of the material required for effective heat storage (the heat of fusion of sodium sulfate decahydrate is 25.53 kJ/mol or about 19 cal/g), with the further advantage of a consistency of temperature as long as sufficient material in the appropriate phase is available.
Small-scale applications
In the laboratory, anhydrous sodium sulfate is widely used as an inert drying agent, for removing traces of water from organic solutions. It is more efficient, but slower-acting, than the similar agent magnesium sulfate. It is only effective below about 30 °C, but it can be used with a variety of materials since it is chemically fairly inert. Sodium sulfate is added to the solution until the crystals no longer clump together; the two video clips (see above) demonstrate how the crystals clump when still wet, but some crystals flow freely once a sample is dry.
Glauber's salt, the decahydrate, was historically used as a laxative. It is effective for the removal of certain drugs such as acetaminophen from the body, for example, after an overdose.
In 1953, sodium sulfate was proposed for heat storage in passive solar heating systems. This takes advantage of its unusual solubility properties, and the high heat of crystallisation (78.2 kJ/mol).
Other uses for sodium sulfate include de-frosting windows, in carpet fresheners, starch manufacture, and as an additive to cattle feed.
Lately, sodium sulfate has been found effective in dissolving very finely electroplated micrometre gold that is found in gold electroplated hardware on electronic products such as pins, and other connectors and switches. It is safer and cheaper than other reagents used for gold recovery, with little concern for adverse reactions or health effects.
At least one company, ThermalTake, makes a laptop computer chill mat (iXoft Notebook Cooler) using sodium sulfate decahydrate inside a quilted plastic pad. The material slowly turns to liquid and recirculates, equalizing laptop temperature and acting as an insulation.
Safety
Although sodium sulfate is generally regarded as non-toxic, it should be handled with care. The dust can cause temporary asthma or eye irritation; this risk can be prevented by using eye protection and a paper mask. Transport is not limited, and no Risk Phrase or Safety Phrase apply.