Copper(II) chloride
About this schools Wikipedia selection
SOS Children volunteers helped choose articles and made other curriculum material Sponsor a child to make a real difference.
| Copper(II) chloride | |
|---|---|
 |
|
 |
|
 |
|
|
Copper(II) chloride |
|
|
Other names
Cupric chloride |
|
| Identifiers | |
| CAS number | 7447-39-4 10125-13-0 (dihydrate) |
| PubChem | 169664 |
| ChemSpider | 148374 |
| UNII | P484053J2Y |
| RTECS number | GL7000000 |
| Jmol-3D images | Image 1 Image 2 |
|
SMILES
|
|
|
InChI
|
|
| Properties | |
| Molecular formula | CuCl2 |
| Molar mass | 134.45 g/mol (anhydrous) 170.48 g/mol (dihydrate) |
| Appearance | yellow-brown solid (anhydrous) blue-green solid (dihydrate) |
| Density | 3.386 g/cm3 (anhydrous) 2.51 g/cm3 (dihydrate) |
| Melting point |
498 °C (anhydrous) |
| Boiling point |
993 °C (anhydrous, decomp) |
| Solubility in water | 70.6 g/100 mL (0 °C) 75.7 g/100 mL (25 °C) |
| Structure | |
| Crystal structure | distorted CdI2 structure |
| Coordination geometry |
Octahedral |
| Hazards | |
| MSDS | Fischer Scientific |
| EU classification | Not listed |
| NFPA 704 | |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Copper(II) fluoride Copper(II) bromide |
| Other cations | Copper(I) chloride Silver chloride Gold(III) chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Copper(II) chloride is the chemical compound with the formula CuCl2. This is a light brown solid, which slowly absorbs moisture to form a blue-green dihydrate. The copper(II) chlorides are some of the most common copper(II) compounds, after copper sulfate.
Structure
Anhydrous CuCl2 adopts a distorted cadmium iodide structure. In this motif, the copper centers are octahedral. Most copper(II) compounds exhibit distortions from idealized octahedral geometry due to the Jahn-Teller effect, which in this case describes the localization of one d-electron into a molecular orbital that is strongly antibonding with respect to a pair of chloride ligands. In CuCl2·2H2O, the copper again adopts a highly distorted octahedral geometry, the Cu(II) centers being surrounded by two water ligands and four chloride ligands, which bridge asymmetrically to other Cu centers.
Copper(II) chloride is paramagnetic. Of historical interest, CuCl2·2H2O was used in the first electron paramagnetic resonance measurements by Yevgeny Zavoisky in 1944.
Properties and reactions
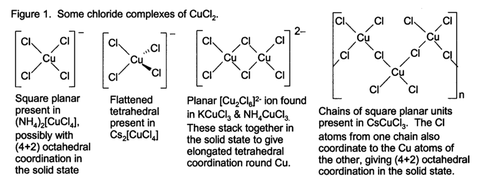
Aqueous solution prepared from copper(II) chloride contain a range of copper(II) complexes depending on concentration, temperature,and the presence of additional chloride ions. These species include blue colour of [Cu(H2O)6]2+ and yellow or red colour of the halide complexes of the formula [CuCl2+x]x−.
It decomposes to CuCl and Cl2 at 1000 °C:
- 2 CuCl2 → 2 CuCl + Cl2
It reacts with HCl or other chloride sources to form complex ions: the red CuCl3−, and the yellow CuCl42−.
- CuCl2 + 2 Cl−
 CuCl−
CuCl−
3 + Cl− CuCl2−
CuCl2−
4
Some of these complexes can be crystallized from aqueous solution, and they adopt a wide variety of structural types (Fig. 1).
Copper(II) hydroxide precipitates upon treating copper(II) chloride solutions with base:
- CuCl2 + 2 NaOH → Cu(OH)2 + 2 NaCl
Copper(II) chloride also forms a variety of coordination complexes with ligands such as pyridine and triphenylphosphine oxide:
- CuCl2 + 2 C5H5N → [CuCl2(C5H5N)2] (tetragonal)
- CuCl2 + 2 (C6H5)3P=O → [CuCl2((C6H5)3P=O)2] (tetrahedral)
However "soft" ligands such as phosphines (e.g., triphenylphosphine), iodide, and cyanide as well as some tertiary amines cause reduction to give copper(I) complexes. To convert copper(II) chloride to copper(I) derivatives it is generally more convenient to reduce an aqueous solution with sulfur dioxide as the reductant:
- 2 CuCl2 + SO2 + 2 H2O → 2 CuCl + 2 HCl + H2SO4
Hydrolysis give the copper oxychloride, Cu2Cl(OH)3, a popular fungicide.
Preparation
Copper(II) chloride is prepared commercially by the action of chlorination of copper:
- Cu + Cl2 + 2 H2O → CuCl2(H2O)2
It can also be generated by treatment of the hydroxide, oxide, or copper(II) carbonate with hydrochloric acid. Electrolysis of aqueous sodium chloride with copper electrodes produces (among other things) a blue-green foam that can be collected and converted to the hydrate.
Anhydrous CuCl2 may be prepared directly by union of the elements, copper and chlorine.
CuCl2 may be purified by crystallization from hot dilute hydrochloric acid, by cooling in a CaCl2-ice bath.
Natural occurrence
Copper(II) chloride occurs naturally as the very rare mineral tolbachite and the dihydrate eriochalcite. Both are found near fumaroles. More common are mixed oxyhydroxide-chlorides like atacamite Cu2(OH)3Cl, arising among Cu ore beds oxidation zones in arid climate (also known from some altered slags).
Uses
Co-catalyst in Wacker Process
A major industrial application for copper(II) chloride is as a co-catalyst with palladium(II) chloride in the Wacker process. In this process, ethene (ethylene) is converted to ethanal (acetaldehyde) using water and air. During the reaction, PdCl2 is reduced to Pd, and the CuCl2 serves to re-oxidize this back to PdCl2. Air can then oxidize the resultant CuCl back to CuCl2, completing the cycle.
- C2H4 + PdCl2 + H2O → CH3CHO + Pd + 2 HCl
- Pd + 2 CuCl2 → 2 CuCl + PdCl2
- 4 CuCl + 4 HCl + O2 → 4 CuCl2 + 2 H2O
The overall process is:
- 2 C2H4 + O2 → 2 CH3CHO
Chlorinations
Copper(II) chloride catalyzes the chlorination in the production of vinyl chloride and dichloroethane.
Other organic synthetic applications
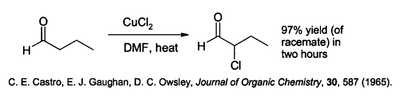
Copper(II) chloride has a variety of specialized applications in the synthesis of organic compounds. It effects chlorination of aromatic hydrocarbons- this is often performed in the presence of aluminium oxide. It is able to chlorinate the alpha position of carbonyl compounds:
This reaction is performed in a polar solvent such as dimethylformamide (DMF), often in the presence of lithium chloride, which accelerates the reaction.
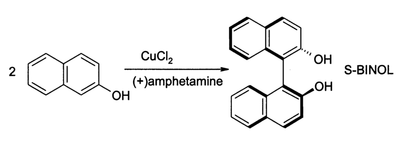
CuCl2, in the presence of oxygen, can also oxidize phenols. The major product can be directed to give either a quinone or a coupled product from oxidative dimerization. The latter process provides a high-yield route to 1,1-binaphthol:
Such compounds are intermediates in the synthesis of BINAP and its derivatives
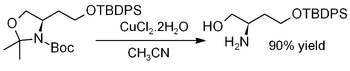
Copper(II) chloride dihydrate promotes the hydrolysis of acetonides, i.e., for deprotection to regenerate diols or aminoalcohols, as in this example (where TBDPS = tert-butyldiphenylsilyl):
CuCl2 also catalyses the free radical addition of sulfonyl chlorides to alkenes; the alpha-chlorosulfone may then undergo elimination with base to give a vinyl sulfone product.
Niche uses
Copper(II) chloride is also used in pyrotechnics as a blue/green coloring agent. In a flame test, copper chlorides, like all copper compounds, emit green-blue.
Safety
It is toxic and only concentrations below 5 ppm are allowed in drinking water by the US Environmental Protection Agency.