|
This is a file from the Wikimedia Commons. Information from its description page there is shown below.
Commons is a freely licensed media file repository. You can help.
|
 |
This image appeared on Wikipedia's Main Page in the Did you know? column on 17 October 2007. |
|
| Description |
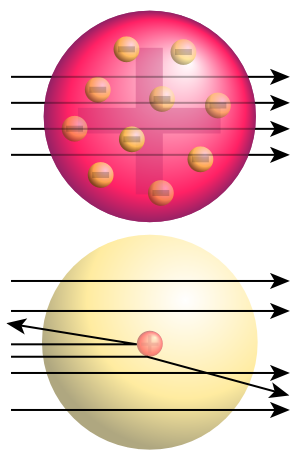
English: Top: Expected results of Rutherford's gold foil experiment: alpha particles passing through the plum pudding model of the atom undisturbed. Bottom: Observed results: Some of the particles were deflected, and some by very large angles. Rutherford concluded that the positive charge of the atom must be concentrated into a very small location: the atomic nucleus.
|
| Date |
April 2008 |
| Source |
Own work |
| Author |
Drawn by User:Fastfission in Illustrator and Inkscape. -- Fastfission 15:04, 14 April 2008 (UTC) |
Permission
( Reusing this file) |
If you want to credit someone, credit "Wikimedia Commons." Otherwise don't credit anyone, that's fine by me.
|
| Public domainPublic domainfalsefalse |
 |
This work has been released into the public domain by its author, Fastfission. This applies worldwide.
In some countries this may not be legally possible; if so:
Fastfission grants anyone the right to use this work for any purpose, without any conditions, unless such conditions are required by law.Public domainPublic domainfalsefalse
|
File usage
The following pages on Schools Wikipedia link to this image (list may be incomplete):
This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file.
Schools Wikipedia was created by children's charity SOS Children's Villages. The world's largest orphan charity, SOS Children brings a better life to more than 2 million people in 133 countries around the globe. Learn more about child sponsorship.