Many organic compounds are closely related to the alkanes. As we noted in Section 12.7 "Chemical Properties of Alkanes", alkanes react with halogens to produce halogenated hydrocarbons, the simplest of which have a single halogen atom substituted for a hydrogen atom of the alkane. Even more closely related are the cycloalkanes, compounds in which the carbon atoms are joined in a ring, or cyclic fashion.
The reactions of alkanes with halogens produce halogenated hydrocarbonsA hydrocarbon in which one or more hydrogen atoms has been replaced by a halogen atom., compounds in which one or more hydrogen atoms of a hydrocarbon have been replaced by halogen atoms:

The replacement of only one hydrogen atom gives an alkyl halide (or haloalkane)A compound resulting from the replacement of a hydrogen atom of an alkane with a halogen atom.. The common names of alkyl halides consist of two parts: the name of the alkyl group plus the stem of the name of the halogen, with the ending -ide. The IUPAC system uses the name of the parent alkane with a prefix indicating the halogen substituents, preceded by number indicating the substituent’s location. The prefixes are fluoro-, chloro-, bromo-, and iodo-. Thus CH3CH2Cl has the common name ethyl chloride and the IUPAC name chloroethane. Alkyl halides with simple alkyl groups (one to four carbon atoms) are often called by common names. Those with a larger number of carbon atoms are usually given IUPAC names.
Give the common and IUPAC names for each compound.
Solution
Give common and IUPAC names for each compound.
CH3CH2I
CH3CH2CH2CH2F
Give the IUPAC name for each compound.
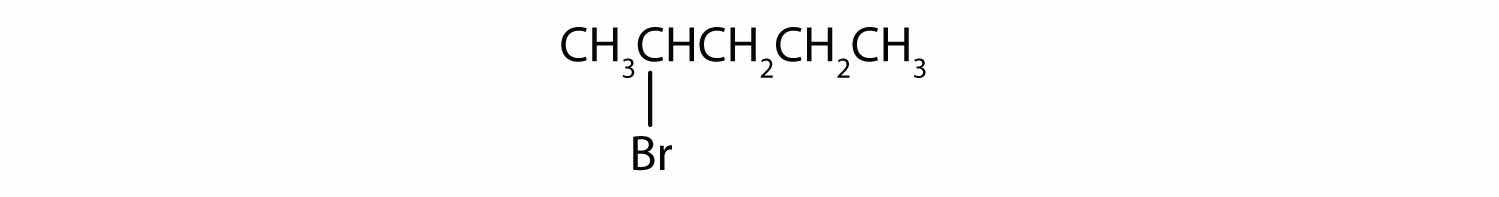
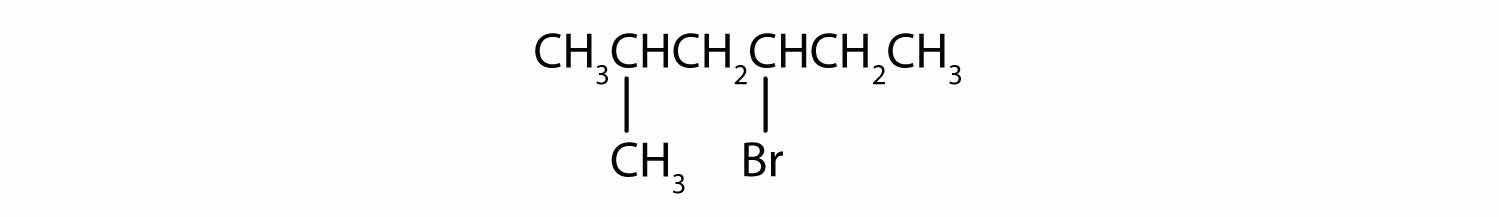
Solution
Give the IUPAC name for each compound.
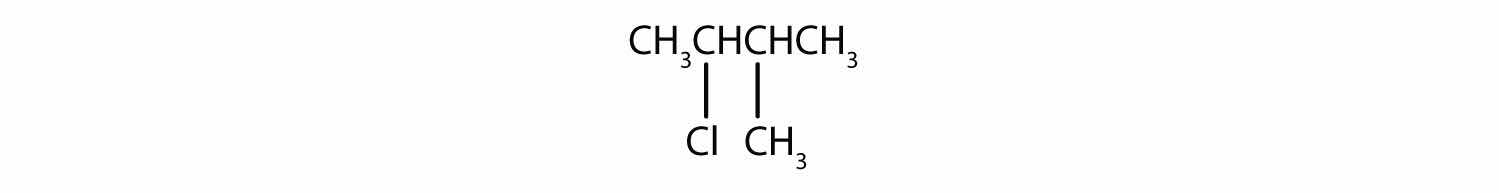
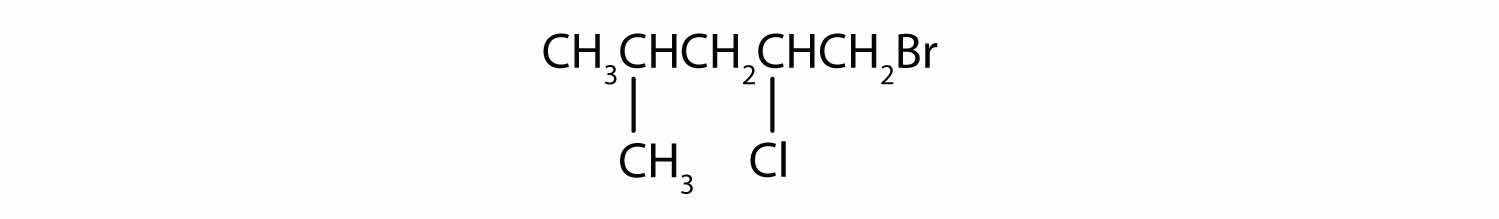
A wide variety of interesting and often useful compounds have one or more halogen atoms per molecule. For example, methane (CH4) can react with chlorine (Cl2), replacing one, two, three, or all four hydrogen atoms with Cl atoms. Several halogenated products derived from methane and ethane (CH3CH3) are listed in Table 12.6 "Some Halogenated Hydrocarbons", along with some of their uses.
Table 12.6 Some Halogenated Hydrocarbons
| Formula | Common Name | IUPAC Name | Some Important Uses |
|---|---|---|---|
| Derived from CH4 | |||
| CH3Cl | methyl chloride | chloromethane | refrigerant; the manufacture of silicones, methyl cellulose, and synthetic rubber |
| CH2Cl2 | methylene chloride | dichloromethane | laboratory and industrial solvent |
| CHCl3 | chloroform | trichloromethane | industrial solvent |
| CCl4 | carbon tetrachloride | tetrachloromethane | dry-cleaning solvent and fire extinguishers (but no longer recommended for use) |
| CBrF3 | halon-1301 | bromotrifluoromethane | fire extinguisher systems |
| CCl3F | chlorofluorocarbon-11 (CFC-11) | trichlorofluoromethane | foaming plastics |
| CCl2F2 | chlorofluorocarbon-12 (CFC-12) | dichlorodifluoromethane | refrigerant |
| Derived from CH3CH3 | |||
| CH3CH2Cl | ethyl chloride | chloroethane | local anesthetic |
| ClCH2CH2Cl | ethylene dichloride | 1,2-dichloroethane | solvent for rubber |
| CCl3CH3 | methylchloroform | 1,1,1-trichloroethane | solvent for cleaning computer chips and molds for shaping plastics |
Once widely used in consumer products, many chlorinated hydrocarbons are suspected carcinogens (cancer-causing substances) and also are known to cause severe liver damage. An example is carbon tetrachloride (CCl4), once used as a dry-cleaning solvent and in fire extinguishers but no longer recommended for either use. Even in small amounts, its vapor can cause serious illness if exposure is prolonged. Moreover, it reacts with water at high temperatures to form deadly phosgene (COCl2) gas, which makes the use of CCl4 in fire extinguishers particularly dangerous.
Ethyl chloride, in contrast, is used as an external local anesthetic. When sprayed on the skin, it evaporates quickly, cooling the area enough to make it insensitive to pain. It can also be used as an emergency general anesthetic.
Bromine-containing compounds are widely used in fire extinguishers and as fire retardants on clothing and other materials. Because they too are toxic and have adverse effects on the environment, scientists are engaged in designing safer substitutes for them, as for many other halogenated compounds.
Alkanes substituted with both fluorine (F) and chlorine (Cl) atoms have been used as the dispersing gases in aerosol cans, as foaming agents for plastics, and as refrigerants. Two of the best known of these chlorofluorocarbons (CFCs) are listed in Table 12.6 "Some Halogenated Hydrocarbons".
Chlorofluorocarbons contribute to the greenhouse effect in the lower atmosphere. They also diffuse into the stratosphere, where they are broken down by ultraviolet (UV) radiation to release Cl atoms. These in turn break down the ozone (O3) molecules that protect Earth from harmful UV radiation. Worldwide action has reduced the use of CFCs and related compounds. The CFCs and other Cl- or bromine (Br)-containing ozone-destroying compounds are being replaced with more benign substances. Hydrofluorocarbons (HFCs), such as CH2FCF3, which have no Cl or Br to form radicals, are one alternative. Another is hydrochlorofluorocarbons (HCFCs), such as CHCl2CF3. HCFC molecules break down more readily in the troposphere, and fewer ozone-destroying molecules reach the stratosphere.
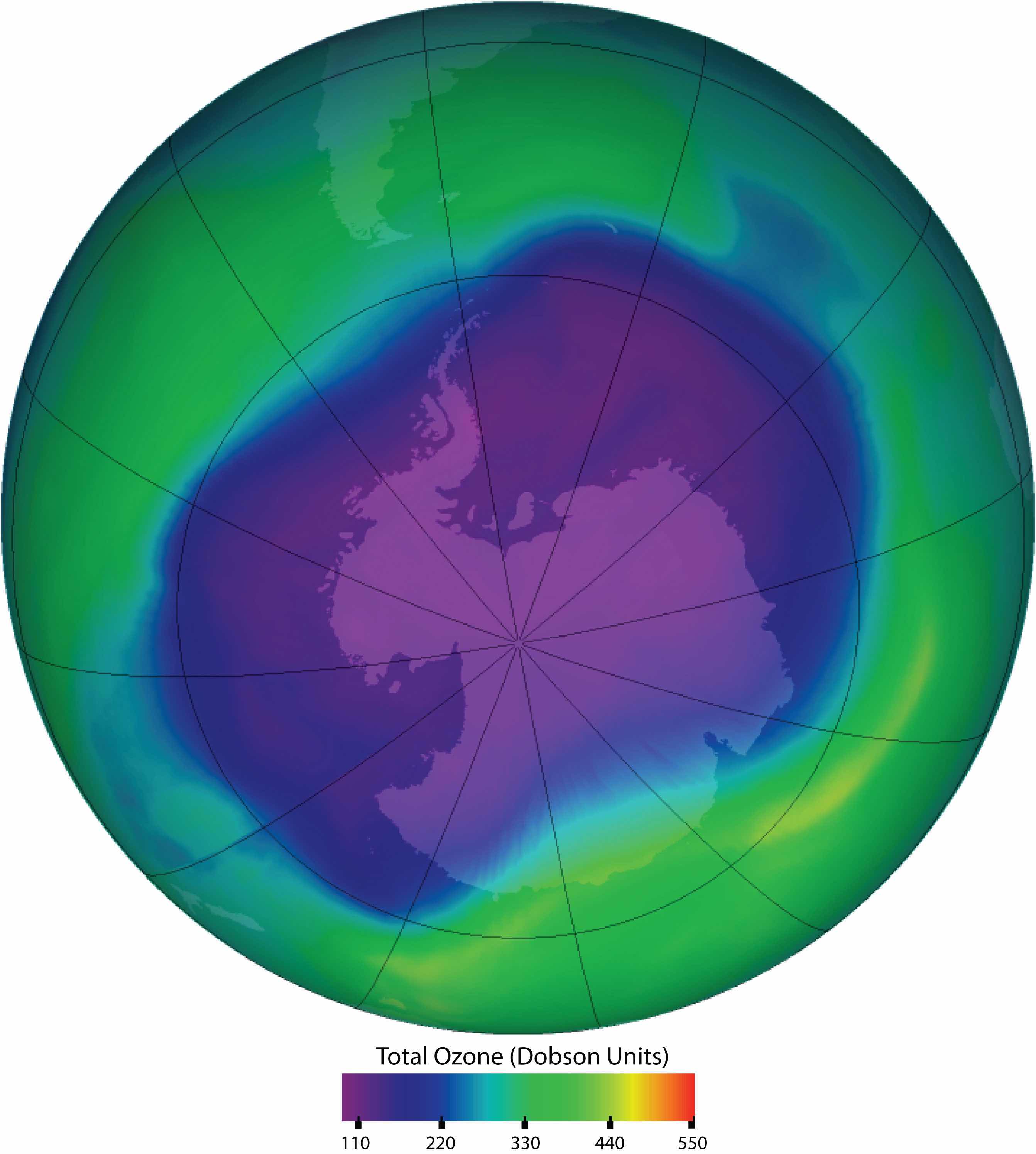
Ozone in the upper atmosphere shields Earth’s surface from UV radiation from the sun, which can cause skin cancer in humans and is also harmful to other animals and to some plants. Ozone “holes” in the upper atmosphere (the gray, pink, and purple areas at the center) are large areas of substantial ozone depletion. They occur mainly over Antarctica from late August through early October and fill in about mid-November. Ozone depletion has also been noted over the Arctic regions. The largest ozone hole ever observed occurred on 24 September 2006.
Source: Image courtesy of NASA, http://ozonewatch.gsfc.nasa.gov/daily.php?date=2006-09-24.
What is the IUPAC name for the HFC that has the formula CH2FCF3? (Hint: you must use a number to indicate the location of each substituent F atom.)
What is the IUPAC name for the HCFC that has the formula CHCl2CF3?
1,1,1,2-tetrafluoroethane
1,1,1-trifluoro-2,2-dichloroethane
Write the condensed structural formula for each compound.
Write the condensed structural formula for each compound.
Write the condensed structural formulas for the two isomers that have the molecular formula C3H7Br. Give the common name and the IUPAC name of each.
Write the condensed structural formulas for the four isomers that have the molecular formula C4H9Br. Give the IUPAC name of each.
What is a CFC? How are CFCs involved in the destruction of the ozone layer?
Explain why each compound is less destructive to the ozone layer than are CFCs.
CH3CH2CH2Br, propyl bromide, 1-bromopropane; CH3CHBrCH3, isopropyl bromide, 2-bromopropane
compounds containing Cl, F, and C; by releasing Cl atoms in the stratosphere