Red blood cells (RBCs) perform a number of human respiratory and cardiovascular system functions. Most of these functions are attributed to hemoglobin content. The main RBC functions are facilitating gas exchange and regulating blood pH.
Gas Exchange
RBCs facilitate gas exchange through a protein called hemoglobin. The word hemoglobin comes from "hemo" meaning blood and "globin" meaning protein. Hemoglobin is a quaternary structure protein consisting of many smaller tertiary structure proteins composed of amino acid polypeptide chains. Each hemoglobin molecule contains four iron-binding heme groups, which are the site of oxygen (O2) binding. Oxygen bound hemoglobin is called oxyhemoglobin.
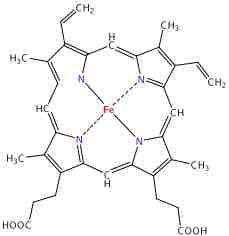
Heme
This is a diagram of the molecular structure of heme.
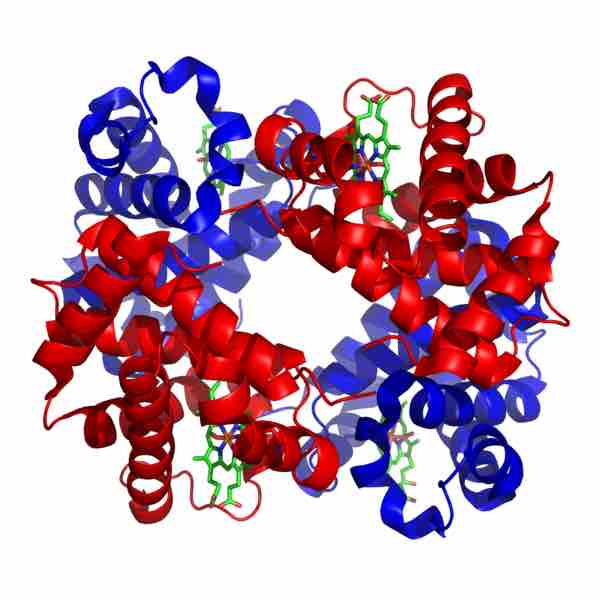
Quaternary structure: hemoglobin
Hemoglobin is a globular protein composed of four polypeptide subunits (two alpha chains, in blue, and two beta pleated sheets, in red). The heme groups are the green structures nestled among the alpha and beta.
The binding of oxygen is a cooperative process. Hemoglobin bound oxygen causes a gradual increase in oxygen-binding affinity until all binding sites on the hemoglobin molecule are filled. As a result, the oxygen-binding curve of hemoglobin (also called the oxygen saturation or dissociation curve) is sigmoidal, or S-shaped, as opposed to the normal hyperbolic curve associated with noncooperative binding. This curve shows the saturation of oxygen bound to hemoglobin compared to the partial pressure of oxygen (concentration) in blood.
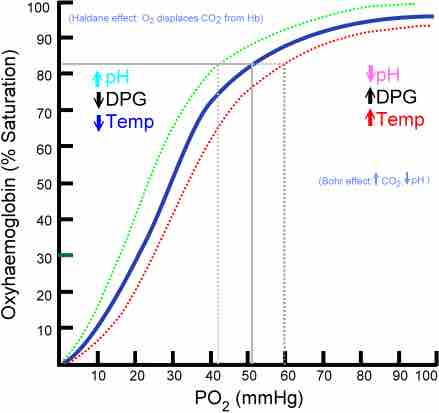
Oxygen saturation curve
Due to cooperative binding, the oxygen saturation curve is S-shaped.
pH Control
RBCs control blood pH by changing the form of carbon dioxide within the blood. Carbon dioxide is associated with blood acidity. That's because most carbon dioxide travels through the blood as a bicarbonate ion, which is the dissociated form of carbonic acid in solution. The respiratory system regulates blood pH by changing the rate at which carbon dioxide is exhaled from the body, which involves the RBC's molecular activity. RBCs alter blood pH in a few different ways.
RBCs secrete the enzyme carbonic anhydrase, which catalyzes the conversion of carbon dioxide and water to carbonic acid. This dissociates in solution into bicarbonate and hydrogen ions, the driving force of pH in the blood. This reaction is reversible by the same enzyme. Carbonic anhydrase also removes water from carbonic acid to turn it back into carbon dioxide and water. This process is essential so carbon dioxide can exist as a gas during gas exchange in the alveolar capillaries. As carbon dioxide is converted from its dissolved acid form and exhaled through the lungs, blood pH becomes less acidic. This reaction can occur without the presence of RBCs or carbonic anhydrase, but at a much slower rate. With the catalyst activity of carbonic anhydrase, this reaction is one of the fastest in the human body.
Hemoglobin can also bind to carbon dioxide, which creates carbamino-hemoglobin. When carbon dioxide binds to hemoglobin, it doesn't exist in the form of carbonic acid, which makes the blood less acidic and increases blood pH. However, because of allosteric effects on the hemoglobin molecule, the binding of carbon dioxide decreases the amount of oxygen bound for a given partial pressure of oxygen. This decrease in hemoglobin's affinity for oxygen by the binding of carbon dioxide is known as the Bohr effect, which results in a rightward shift to the O2-saturation curve. Conversely, when the carbon dioxide levels in the blood decrease (i.e., in the lung capillaries), carbon dioxide and hydrogen ions are released from hemoglobin, increasing the oxygen affinity of the protein. A reduction in the total binding capacity of hemoglobin to oxygen (i.e. shifting the curve down, not just to the right) due to reduced pH is called the Haldane effect.