Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle that consists of two protons and two neutrons, as shown in . As the result of this process, the parent atom transforms ("decays") into a new atom with a mass number smaller by four and an atomic number smaller by two.
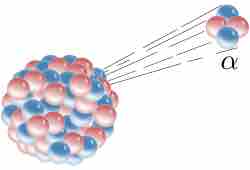
Alpha Decay
Alpha decay is one type of radioactive decay. An atomic nucleus emits an alpha particle and thereby transforms ("decays") into an atom with a mass number smaller by four and an atomic number smaller by two. Many other types of decay are possible.
For example: 238U → 234Th + α
Because an alpha particle is the same as a helium-4 nucleus, which has mass number 4 and atomic number 2, this can also be written as:
238
92U → 234
90Th + 4
2He
The alpha particle also has charge +2, but the charge is usually not written in nuclear equations, which describe nuclear reactions without considering the electrons. This convention is not meant to imply that the nuclei necessarily occur in neutral atoms.
Alpha decay is by far the most common form of cluster decay, in which the parent atom ejects a defined daughter collection of nucleons, leaving another defined product behind (in nuclear fission, a number of different pairs of daughters of approximately equal size are formed). Alpha decay is the most common cluster decay because of the combined extremely high binding energy and relatively small mass of the helium-4 product nucleus (the alpha particle).
Alpha decay typically occurs in the heaviest nuclides. In theory it can occur only in nuclei somewhat heavier than nickel (element 28), in which overall binding energy per nucleon is no longer a minimum and the nuclides are therefore unstable toward spontaneous fission-type processes. The lightest known alpha emitters are the lightest isotopes (mass numbers 106-110) of tellurium (element 52).
Alpha particles have a typical kinetic energy of 5 MeV (approximately 0.13 percent of their total energy, i.e., 110 TJ/kg) and a speed of 15,000 km/s. This corresponds to a speed of around 0.05 c. There is surprisingly small variation in this energy, due to the heavy dependence of the half-life of this process on the energy produced.
Because of their relatively large mass, +2 electric charge, and relatively low velocity, alpha particles are very likely to interact with other atoms and lose their energy, so their forward motion is effectively stopped within a few centimeters of air.
Most of the helium produced on Earth (approximately 99 percent of it) is the result of the alpha decay of underground deposits of minerals containing uranium or thorium. The helium is brought to the surface as a byproduct of natural gas production.