Pressure is defined in simplest terms as force per unit area. However, when dealing with pressures exerted by gases and liquids, it is most convenient to approach pressure as a measure of energy per unit volume by means of the definition of work (W = F·d). The derivation of pressure as a measure of energy per unit volume from its definition as force per unit area is given in . Since, for gases and liquids, the force acting on a system contributing to pressure does not act on a specific point or particular surface, but rather as a distribution of force, analyzing pressure as a measure of energy per unit volume is more appropriate. For liquids and gases at rest, the pressure of the liquid or gas at any point within the medium is called the hydrostatic pressure. At any such point within a medium, the pressure is the same in all directions, as if the pressure was not the same in all directions, the fluid, whether it is a gas or liquid, would not be static. Note that the following discussion and expressions pertain only to incompressible fluids at static equilibrium.
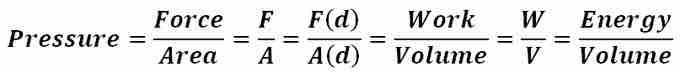
Energy per Unit Volume
This equation is the derivation of pressure as a measure of energy per unit volume from its definition as force per unit area.
The pressure exerted by a static liquid depends only on the depth, density of the liquid, and the acceleration due to gravity. gives the expression for pressure as a function of depth within an incompressible, static liquid as well as the derivation of this equation from the definition of pressure as a measure of energy per unit volume (ρ is the density of the gas, g is the acceleration due to gravity, and h is the depth within the liquid). For any given liquid with constant density throughout, pressure increases with increasing depth. For example, a person under water at a depth of h1 will experience half the pressure as a person under water at a depth of h2 = 2h1. For many liquids, the density can be assumed to be nearly constant throughout the volume of the liquid and, for virtually all practical applications, so can the acceleration due to gravity (g = 9.81 m/s2). As a result, pressure within a liquid is therefore a function of depth only, with the pressure increasing at a linear rate with respect to increasing depth. In practical applications involving calculation of pressure as a function of depth, an important distinction must be made as to whether the absolute or relative pressure within a liquid is desired. Equation 2 by itself gives the pressure exerted by a liquid relative to atmospheric pressure, yet if the absolute pressure is desired, the atmospheric pressure must then be added to the pressure exerted by the liquid alone.
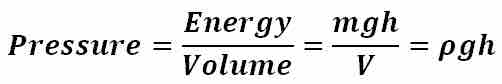
Pressure as Energy per Unit Volume
This equation gives the expression for pressure as a function of depth within an incompressible, static liquid as well as the derivation of this equation from the definition of pressure as a measure of energy per unit volume (ρ is the density of the gas, g is the acceleration due to gravity, and h is the depth within the liquid).
When analyzing pressure within gases, a slightly different approach must be taken as, by the nature of gases, the force contributing to pressure arises from the average number of gas molecules occupying a certain point within the gas per unit time. Thus the force contributing to the pressure of a gas within the medium is not a continuous distribution as for liquids and the barometric equation given in must be utilized to determine the pressure exerted by the gas at a certain depth (or height) within the gas (p0 is the pressure at h = 0, M is the mass of a single molecule of gas, g is the acceleration due to gravity, k is the Boltzmann constant, T is the temperature of the gas, and h is the height or depth within the gas). Equation 3 assumes that the gas is incompressible and that the pressure is hydrostatic.
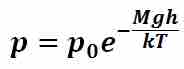
Pressure within a gas
The force contributing to the pressure of a gas within the medium is not a continuous distribution as for liquids and the barometric equation given in this figure must be utilized to determine the pressure exerted by the gas at a certain depth (or height) within the gas (p0 is the pressure at h = 0, M is the mass of a single molecule of gas, g is the acceleration due to gravity, k is the Boltzmann constant, T is the temperature of the gas, and h is the height or depth within the gas)