Concept
Version 7
Created by Boundless
Polarization
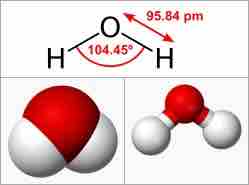
Water Molecule
Water is an example of a dipole molecule, which has a bent shape (the H-O-H angle is 104.45°) and in which the oxygen pulls electron density away from the H atoms, leaving the H relatively positive and the O relatively negative.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: