In anaerobic respiration, denitrification utilizes nitrate (NO3-) as a terminal electron acceptor in the respiratory electron transport chain. Denitrification is a widely used process; many facultative anaerobes use denitrification because nitrate, like oxygen, has a high reduction potential
Denitrification is a microbially facilitated process involving the stepwise reduction of nitrate to nitrite (NO2-) nitric oxide (NO), nitrous oxide (N2O), and, eventually, to dinitrogen (N2) by the enzymes nitrate reductase, nitrite reductase, nitric oxide reductase, and nitrous oxide reductase. The complete denitrification process can be expressed as a redox reaction: 2 NO3− + 10 e− + 12 H+ → N2 + 6 H2O.
Protons are transported across the membrane by the initial NADH reductase, quinones and nitrous oxide reductase to produce the electrochemical gradient critical for respiration. Some organisms (e.g. E. coli) only produce nitrate reductase and therefore can accomplish only the first reduction leading to the accumulation of nitrite. Others (e.g. Paracoccus denitrificans or Pseudomonas stutzeri) reduce nitrate completely. Complete denitrification is an environmentally significant process because some intermediates of denitrification (nitric oxide and nitrous oxide) are significant greenhouse gases that react with sunlight and ozone to produce nitric acid, a component of acid rain. Denitrification is also important in biological wastewater treatment, where it can be used to reduce the amount of nitrogen released into the environment, thereby reducing eutrophication.
Denitrification takes place under special conditions in both terrestrial and marine ecosystems. In general, it occurs where oxygen is depleted and bacteria respire nitrate as a substitute terminal electron acceptor. Due to the high concentration of oxygen in our atmosphere, denitrification only takes place in anaerobic environments where oxygen consumption exceeds the oxygen supply and where sufficient quantities of nitrate are present. These environments may include certain soils and groundwater, wetlands, oil reservoirs, poorly ventilated corners of the ocean, and in sea floor sediments.
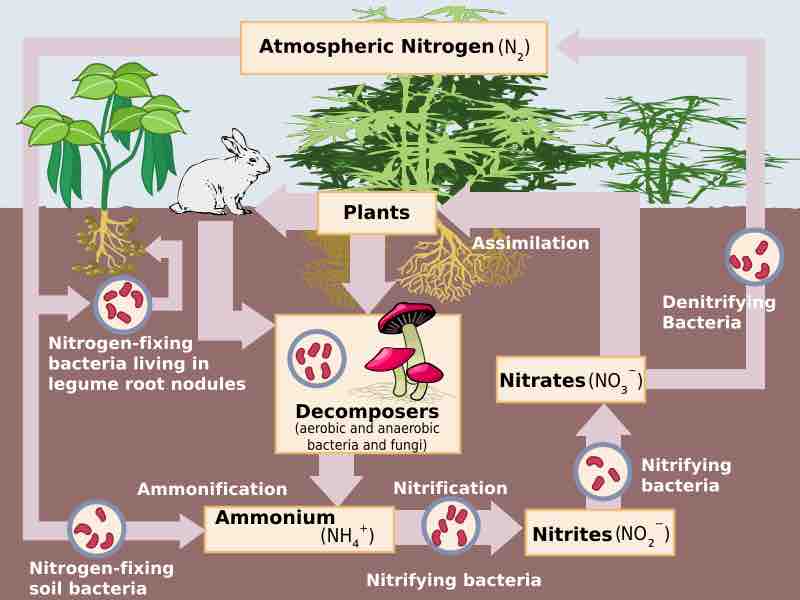
The role of soil bacteria in the Nitrogen cycle
Denitrification is an important process in maintaining ecosystems. Generally, denitrification takes place in environments depleted of oxygen.
Denitrification is performed primarily by heterotrophic bacteria (e.g. Paracoccus denitrificans), although autotrophic denitrifiers have also been identified (e.g., Thiobacillus denitrificans). Generally, several species of bacteria are involved in the complete reduction of nitrate to molecular nitrogen, and more than one enzymatic pathway have been identified in the reduction process.
Rhizobia are soil bacteria with the unique ability to establish a N2-fixing symbiosis on legume roots. When faced with a shortage of oxygen, some rhizobia species are able to switch from O2-respiration to using nitrates to support respiration.
The direct reduction of nitrate to ammonium (dissimilatory nitrate reduction) can be performed by organisms with the nrf-gene. This is a less common method of nitrate reduction than denitrification in most ecosystems. Other genes involved in denitrification include nir (nitrite reductase) and nos (nitrous oxide reductase), which are possessed by such organisms as Alcaligenes faecalis, Alcaligenes xylosoxidans, Pseudomonas spp, Bradyrhizobium japonicum, and Blastobacter denitrificans.