Cancer immunotherapy is the use of the body's own immune system to reject cancer. The main idea is stimulating the patient's immune system to attack the malignant tumor cells that are responsible for the disease. This can be either through immunization of the patient (e.g., by administering a cancer vaccine such as Dendreon's Provenge), in which case the patient's own immune system is trained to recognize tumor cells as targets to be destroyed, or through the administration of therapeutic antibodies as drugs, in which case the patient's immune system is recruited to destroy tumor cells by the therapeutic antibodies. Cell-based immunotherapy is another major entity of cancer immunotherapy. This involves immune cells such as the natural killer cells (NK cells), lymphokine-activated killer cells (LAK cells), cytotoxic T lymphocytes (CTLs), and dendritic cells (DC). These immune cells are either activated in vivo by administering certain cytokines such as interleukins, or they are isolated, enriched, and transfused back into the patient to fight against cancer.
Since the immune system responds to the environmental factors it encounters on the basis of discrimination between self and non-self, many kinds of tumor cells that arise as a result of the onset of cancer are more or less tolerated by the patient's own immune system since the tumor cells are essentially the patient's own cells that are growing, dividing, and spreading without proper regulatory control. In spite of this fact, however, many kinds of tumor cells display unusual antigens that are inappropriate for either the cell type or its environment or that are only normally present during the organism's development (e.g. fetal antigens). Examples of such antigens include the glycosphingolipid GD2 , a disialoganglioside that is normally only expressed at a significant level on the outer surface membranes of neuronal cells, where its exposure to the immune system is limited by the blood-brain barrier.
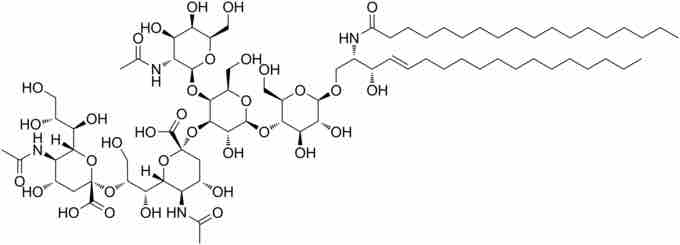
Structure of GD2
GD2 is a disialoganglioside expressed on tumors of neuroectodermal origin, including human neuroblastomas and melanomas, with highly restricted expression on normal tissues, principally to the cerebellum and peripheral nerves in humans. The relatively tumor-specific expression of GD2 makes it a suitable target for immunotherapy with monoclonal antibodies or with artificial T-cell receptors.
GD2 is expressed on the surfaces of a wide range of tumor cells, including neuroblastomas, medulloblastomas, astrocytomas, melanomas, small-cell lung cancer, osteosarcomas, and other soft tissue sarcomas. GD2 is thus a convenient tumor-specific target for immunotherapies.
Adoptive cell-based immunotherapy involves isolating either allogenic or autologous immune cells, enriching them outside the body, and transfusing them back to the patient. The injected immune cells are highly cytotoxic to the cancer cells and so help to fight them.
Antibodies are a key component of the adaptive immune response. They play a central role in both the recognition of foreign antigens and the stimulation of an immune response to them. It is not surprising, therefore, that many immunotherapeutic approaches involve the use of antibodies. The advent of monoclonal antibody technology has made it possible to raise antibodies against specific antigens, such as the unusual antigens that are presented on the surfaces of tumors. A number of therapeutic monoclonal antibodies have been approved for use in humans, such as alemtuzumab, an anti-CD52 humanized IgG1 monoclonal antibody indicated for the treatment of chronic lymphocytic leukemia (CLL). Radioimmunotherapy in turn involves the use of radioactively conjugated murine antibodies against cellular antigens, mostly for treatment of lymphomas.
The development and testing of second-generation immunotherapies is already under way. While antibodies targeted to disease-causing antigens can be effective under certain circumstances, in many cases their efficacy may be limited by other factors. In the case of cancer tumors, the microenvironment is immunosuppressive, allowing even those tumors that present unusual antigens to survive and flourish in spite of the immune response generated by the cancer patient against his own tumor tissue. Certain members of a group of molecules known as cytokines, such as interleukin-2, also play a key role in modulating the immune response. Cytokines have been tested in conjunction with antibodies in order to generate an even more devastating immune response against the tumor. While the therapeutic administration of such cytokines may cause systemic inflammation, resulting in serious side effects and toxicity, there is a new generation of chimeric molecules consisting of an immune-stimulatory cytokine attached to an antibody that targets a tumor. These chimeric molecules are able to generate a very effective yet localized immune response against the tumor tissue, destroying the cancer-causing cells without the unwanted side effects.
Dermatologists use new creams and injections in the management of benign and malignant skin tumors. Topical immunotherapy utilizes an immune enhancement cream (imiquimod), which is an interferon producer, causing the patient's own killer T cells to destroy warts, actinic keratoses, basal cell carcinoma, squamous cell carcinoma, cutaneous T cell lymphoma, and superficial spreading melanoma. Injection immunotherapy uses mumps, candida, or trichophytin antigen injections to treat warts (HPV-induced tumors).