Complement fixation is a classic method for demonstrating the presence of antibody in patient serum. The complement fixation test consists of two components. The first component is an indicator system that uses combination of sheep red blood cells, complement-fixing antibody such as immunoglobulin G produced against the sheep red blood cells and an exogenous source of complement usually guinea pig serum. When these elements are mixed in optimum conditions, the anti-sheep antibody binds on the surface of red blood cells. Complement subsequently binds to this antigen-antibody complex formed and will cause the red blood cells to lyse .
The complement pathway
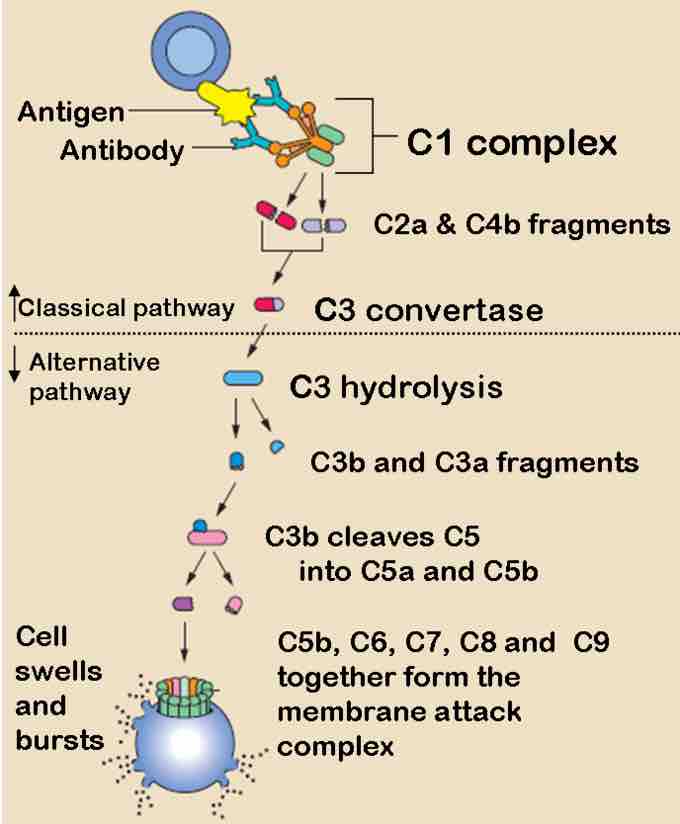
Complement binds to antigen-antibody complex and leads to cell lysis.
The second component is a known antigen and patient serum added to a suspension of sheep red blood cells in addition to complement. These two components of the complement fixation method are tested in sequence. Patient serum is first added to the known antigen, and complement is added to the solution. If the serum contains antibody to the antigen, the resulting antigen-antibody complexes will bind all of the complement. Sheep red blood cells and the anti-sheep antibody are then added. If complement has not been bound by an antigen-antibody complex formed from the patient serum and known antigens, it is available to bind to the indicator system of sheep cells and anti-sheep antibody. Lysis of the indicator sheep red blood cells signifies both a lack of antibody in patient serum and a negative complement fixation test. If the patient's serum does contain a complement-fixing antibody, a positive result will be indicated by the lack of red blood cell lysis.